
Overview
The National Institutes of Health (NIH) is collaborating with the Foundation for NIH (FNIH), the Food and Drug Administration (FDA), pharmaceutical and biotechnology companies, and non-profit organizations to accelerate the identification of new and effective therapies for people living with amyotrophic lateral sclerosis (ALS) or at risk for developing ALS. The AMP® program incorporates several disease areas including Parkinson’s disease (AMP® PD), Alzheimer’s disease (AMP® AD), amyotrophic lateral sclerosis (AMP® ALS), and several others with the common goal of identifying clinically relevant disease targets, improving identification of patients most likely to respond to a particular treatment, and safely and effectively reducing the development timelines for life-saving therapies and improvements in patient outcomes.
About Amyotrophic Lateral Sclerosis (ALS)
ALS is a rapidly progressing, neurodegenerative disease that typically occurs in mid-life, causing weakness and wasting of most skeletal muscles including the diaphragm, as well as cognitive and behavioral changes in about a third of cases. Most people affected by ALS succumb to the disease within 3-5 years of symptom onset. According to the National ALS Registry, there are approximately 30,000 people living with ALS in the United States, with 5,000 new patients on average diagnosed annually. About 90% of cases occur without any known family history or genetic cause, also known as “sporadic” ALS. The remaining 10% of cases occur within families, known as “familial” ALS.
The causes of ALS are unknown and continue to be highly studied. Ongoing research seeks to understand how different factors, such as a person's genetic makeup, as well as external factors and processes, contribute to the risk and onset of ALS, and how to treat and cure it. Scientists are studying cellular defects, genetics and epigenetics, and biomarkers to better understand the progression and mechanisms of the disease, detect its onset and measure its progression.
Need for New Therapies
To date, treatment options for ALS remain severely limited and have modest benefits. No known treatments can halt or reverse neurodegeneration, which is why there is an urgent and unmet need to develop more effective treatments for ALS. Guided by input from people with lived experience of ALS and the ALS advocacy community, AMP® ALS has a common goal of establishing a comprehensive strategy to expedite the development of effective new treatments for people living with ALS, or at risk for developing ALS.
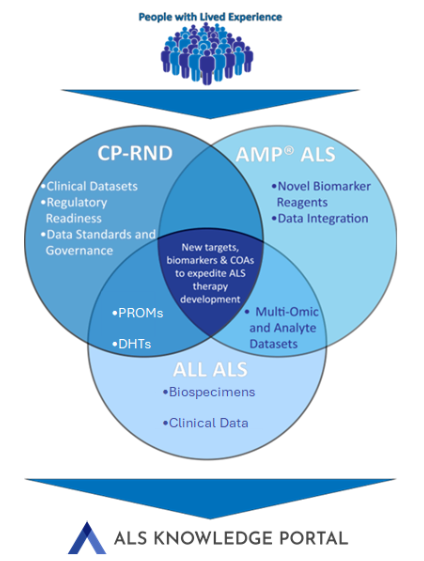
AMP® ALS Approach
AMP® ALS aims to accelerate therapeutic and biomarker development for familial and sporadic ALS by addressing the challenges of disease heterogeneity, segmentation of research efforts, decentralized data storage, and a lack of standardized clinical tools. Through this public-private partnership, the current model for development of new diagnostics and treatments is being transformed by jointly identifying and validating promising biomarkers of disease, new and optimized clinical outcome assessments, and biological targets for therapeutics. By bringing together data and biospecimens collected in the Access for All (ALL) in ALS Clinical Research Consortium, ACT for ALS expanded access research studies, and extant clinical, molecular, and genetic data sets, and making this information available via the openly accessible ALS Knowledge Portal and biorepositories, researchers in academia and industry conducting AMP® ALS-specific projects and the broader research community will be better positioned to identify novel research and therapeutic targets.
Goals
AMP® ALS has developed a set of goals to advance the objectives of this program.
- Establish a central ALS Knowledge Platform for data sharing and analysis through a cloud-based infrastructure
- Develop validated biomarkers for early diagnosis and treatment assessment
- Improve clinical outcome assessments
- Discover new therapeutic targets and risk factors
PWLE Involvement and ALS Advocacy Community
People with lived experience (PWLE) are central to AMP® ALS project. They include those diagnosed with ALS or at genetic risk, loved ones, caregivers, and those who have lost loved ones to ALS. PWLE of ALS are included in all aspects of AMP® ALS, from design to implementation. ALS research advocates and representatives of ALS organizations with significant reach and community perspective work alongside PWLE to contribute their expertise to various areas within the AMP® ALS project. During the project design phase and continuing into the implementation phase, PWLE and ALS advocates serve in technical working groups, along with select staff from NIH, FNIH, FDA, and C-Path. The active participation and input from PWLE and the ALS advocacy community in these working groups contributes to the trajectory of the work of AMP® ALS in several ways.
Governance
The steering committee (SC) for AMP® ALS consists of representatives from each of the research partner organizations. The SC operates under the direction of the overall AMP® Executive Committee and is organized by the FNIH. The AMP® ALS SC considers recommendations developed by various working groups that address a range of scientific and administrative topics related to the implementation of the project's research objectives.
Partners and Collaborators
The Public-Private Partnership is bringing together researchers, funders, and people with lived experience to accelerate therapy development for ALS. As conveners of AMP® ALS, the Critical Path Institute (C-Path) and FNIH provide overall organizational structure, raise private funds, manage resources, and facilitate regulatory endorsement of methods and tools. Public and private partners contribute funds or in-kind resources and are vital in the development of project objectives and strategy. ALS and research experts including the PWLE community, advocates, clinicians, academic and industry researchers provide information to guide decisions and conduct research studies which contribute to data/biospecimen collection, storage, dissemination and analysis.
| Government | Non-profit Organizations | Industry |
|---|---|---|
| NIH-NINDS | Foundation for NIH (FNIH) | Abbvie |
| Food and Drug Administration (FDA) | Critical Path Institute (C-Path) | Biogen |
| ALS Association | Eli Lilly | |
| ALS Therapy Development Institute | EMD Serono | |
| ALS United | GSK | |
| Answer ALS | Mitsubishi Tanabe Pharma America, Inc. | |
| The Association for Frontotemporal Degeneration | QurAlis | |
| Target ALS |
NINDS Program Contacts for AMP® ALS
| Name | Role | Areas of Interest |
|---|---|---|
| Amelie Gubitz, Ph.D. | Lead Program Director for ALS | ALS basic, translational, and clinical research; ALS Public-Private Partnership |
| Frank Shewmaker, Ph.D. | Program Director | ALS Public-Private Partnership |
| Christine Swanson-Fischer, Ph.D. | Program Director | ALS Public-Private Partnership |
| Srikanth Ranganathan, Ph.D. | Program Director | ALS Public-Private Partnership |
| Lumy Sawaki-Adams, MD, Ph.D. | Program Director | Clinical research, including natural history & clinical trials |
| Amy Y. Tsou, M.D., M.Sc. | Program Director | Clinical research and outcomes |
| Dina M. Lyon, M.S., R.N. | Clinical Research Project Manager | Neurodegenerative disease, neuromuscular disorders |
| Thomas Taetzsch, Ph.D. | Health Program Specialist | ALS basic, translational, and clinical research; ALS Public-Private Partnership |
| Elio Peraza, M.Sc. | ALS Program Coordinator | Planning, coordination, management of ALS-related efforts, and engagement with the PWLE community |
Accelerating Medicines Partnership® Parkinson's Disease (AMP® PD)
Accelerating Medicines Partnership® Autoimmune and Immune-Mediated Diseases (AMP® AIM)
Accelerating Medicines Partnership® Program for Alzheimer’s Disease (AMP® AD 1.0)
Accelerating Medicines Partnership® Program for Alzheimer’s Disease (AMP® AD 2.0)
Accelerating Medicines Partnership® Bespoke Gene Therapy Consortium (AMP® BGTC)
Accelerating Medicines Partnership® Common Metabolic Diseases (AMP® CMD)
Accelerating Medicines Partnership® Heart Failure (AMP® HF)
Accelerating Medicines Partnership® Schizophrenia (AMP® SCZ)
