
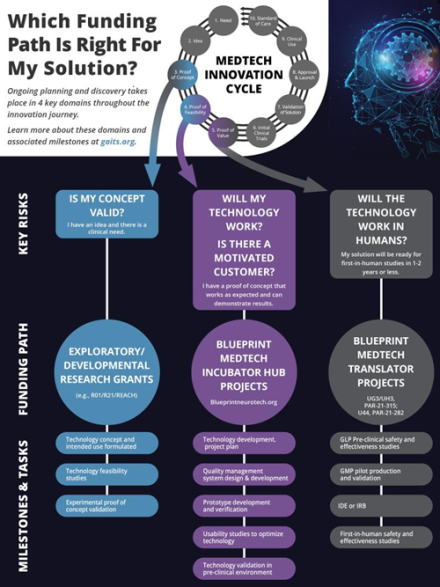
The Blueprint MedTech program is an NIH incubator that aims to support the innovators by accelerating the development of cutting-edge medical devices for diagnosing and treating disorders of the nervous system. The mission of the program is to catalyze the translation of novel technologies from early-stage development to readiness for first-in-human clinical studies. By providing non-dilutive funding and access to essential resources, including planning support, design and testing services, and expert consultations on regulatory and commercialization issues, the program seeks to facilitate the translation of novel technologies to readiness for first-in-human clinical studies. Its goal is to enhance patient access to safe and effective medical devices while attracting further investment from industry and government.
MedTech Innovation Cycle
Program Structure
The Blueprint MedTech Program is structured in two main components:
Blueprint MedTech Incubator
This program will catalyze the translation of novel technologies from early-stage development up to readiness for first-in-human clinical studies. Funding for late-stage development as Optimizer projects would cover up to $500,000 in direct costs per year for up to four years; Sprinter awards provide support for six months, a $25,000 stipend, and $25,000 to hire subject matter experts. For these, mentors will work with awardees to help resolve specifically identified gap(s) on the path to commercialization.
We recommend applicants first look at NIH Institute/Center specific interests to align their application with NIH interests.
Please visit the Blueprint Medtech Incubator Solicitation page.
Blueprint MedTech Translator
The purpose of these Notices of Funding Opportunities (NOFO) is to encourage investigators (UG3/UH3 mechanism) to pursue translational activities and clinical feasibility studies to advance the development of therapeutic and diagnostic devices for disorders that affect the nervous or neuromuscular systems.

BP MedTech Participating Institutes/Centers and Programs
The following Institutes, Centers, and Offices (ICs) will be participating in the Blueprint MedTech program. Institute specific interests are also provided below. Interested applicants are encouraged to contact the NIH Scientific/Research IC program staff for more information about this program before submitting an application.
Brain Research Through Advancing Innovative Neurotechnologies® (BRAIN) Initiative
Contact: Nick Langhals, Ph.D. and Megan Frankowski, Ph.D.
There is a need to help transition BRAIN Initiative-relevant technologies from early device development to first-in-human studies. Projects that fit the following categories can be submitted through the Blueprint MedTech Program. BRAIN will support the following efforts coming into the BP MedTech UG3/UH3 funding opportunity:
- Projects developing novel invasive neurostimulation devices for the human central nervous system (CNS).
- Projects developing novel invasive brain recording devices for the human CNS.
- Projects developing novel non-invasive brain stimulation devices for the human CNS. These devices should aim to achieve brain stimulation resolution of sub-millimeter at the cortical surface and depth. These approaches may incorporate electromagnetic, mechanical, or combination biological and device means (e.g., optogenetics).
For more information, see the Notice of Shared Interest (NOSI) NOT-NS-22-052.
National Center for Medical Rehabilitation Research (NCMRR)
Contact: Theresa Cruz, Ph.D., Toyin Ajisafe, Ph.D.
The National Center for Medical Rehabilitation Research within NICHD supports assistive and rehabilitation technology to improve the function of people with physical disabilities. NICHD will only accept applications related to the mission of the National Center for Medical Rehabilitation Research.
Helping to End Addiction Long-term (HEAL) Initiative
Contact: Eric Hudak, Ph.D.
Through Notice of Special Interest NOT-NS-24-075, the NIH HEAL Initiative encourages the development and translation of novel neurotechnologies, funded through the Helping to End Addiction Long-term (HEAL) Initiative and overseen by the NIH Blueprint MedTech program. Academic institutions and Small Business Concerns (SBCs) are encouraged to submit grant applications that propose non-clinical development and validation activities for subsequent clinical feasibility studies of medical devices for the diagnosis and treatment of pain and opioid use disorder (OUD). Applications supporting the development and translation of groundbreaking neurotechnologies that fit within the mission of the HEAL Initiative are encouraged.
For more information, see HEAL’s Notice of Special Interest here: NOT-NS-24-075
National Center for Complementary and Integrative Health (NCCIH)
Contact: Emrin Horgusluoglu, Ph.D.
The National Center for Complementary and Integrative Health (NCCIH) supports the development and validation of technologies that can facilitate the integration of complementary and integrative health approaches to enhance diagnosis, prevention, or treatment of diseases and/or associated symptoms, or promotion of well-being and whole person health relevant to the nervous and neuromuscular systems. In addition, NCCIH supports the integration of technologies with multisystem studies to understand the connections and interactions across systems involving the brain and the rest of the nervous system such as interoception, and/or the impact of multi-component interventions on multisystem connections and interactions in pre-clinical models or human subjects. NCCIH will not support clinical efficacy studies or pivotal trials of an intervention.
Complementary health approaches include a broad range of practices and interventions that are not typically part of conventional medical care. They can be classified by their primary therapeutic input, including nutritional (e.g., special diets, dietary supplements, herbs, probiotics, and microbial-based therapies), psychological (e.g., meditation, hypnosis, music-based interventions, relaxation therapies), physical (e.g., acupuncture, massage, chiropractic manipulation, other force-based manipulations, or devices related to these approaches), or a combination of psychological and physical (e.g., yoga, tai chi, dance therapies, some forms of art therapy such as music-based interventions).
National Eye Institute (NEI)
Contact: Tony Gover, Ph.D.; Paek Lee, Ph.D.
The National Eye Institute is requesting applications for the development of FDA Class III medical devices, as well as invasive ocular implants and prosthetics (retinal or cortical) that can stimulate retinal or cortical neurons to produce visual percepts. NEI is not interested in projects focused on developing diagnostic/imaging devices or assistive devices through this program.
National Institute of Biomedical Imaging and Bioengineering (NIBIB)
Contact: Michael Wolfson, Ph.D.; Stacie Gutowski, Ph.D.
The mission of the National Institute of Biomedical Imaging and Bioengineering (NIBIB) is to transform, through technology development, our understanding of disease and its prevention, detection, diagnosis, and treatment. NIBIB may support the development of broadly applicable products, where the disease or organ being targeted is used as an initial model and could be adapted to other indications in the future. Before contacting NIBIB, applicants should first discuss the initial target with the Institute and/or Center on this page that is most relevant to the disease(s) being addressed by the proposed product.
National Institute of Dental and Craniofacial Research (NIDCR)
Contact: Melissa Ghim, Ph.D.; Margaret Grisius, D.D.S.; Lorena Baccaglini, D.D.S., Ph.D.
NIDCR is interested in:
- Development of technologies for oral somatosensory or autonomic nerve stimulation to enable diagnosis and/or treatment of motor and sensory conditions, such as bruxism, sleep apnea, temporomandibular/facial pain, swallowing reflex, salivary gland production, and other dental, oral, and craniofacial related conditions and disorders related to the nervous system. These technologies can include the integration of intra- and extra-oral sensors and relevant treatment delivery mechanisms controlled by software systems that allow capture, analysis and display of target biosignatures including but not limited to neural activity.
- Biofeedback & multimodal neurofeedback technologies for treatment of facial nerve disorders, as well as neurological (e.g., trigeminal neuralgia, peripheral neuropathy associated with Sjogren’s syndrome, burning mouth syndrome) and non-neurological (e.g., vascular/muscular, immune) facial pain. These technologies can include wearable and embeddable devices as well as virtual or augmented reality technology to address acute and chronic conditions.
- Development, validation and testing of novel technologies to promote prevention and treatment of orofacial and craniofacial nerve injuries, including nerve regeneration.
- Development, validation and testing of technologies that improve the accuracy and validity of dental, oral or craniofacial clinical pain measurements.
NIDCR will not support any projects that are not directly relevant to the NIDCR mission. Projects that shift away from the NIDCR mission will no longer receive funding.
National Institute of Mental Health (NIMH)
Contact: Eunyoung Kim, Ph.D.; Margaret (Meg) Grabb, Ph.D.
NIMH is specifically interested in novel brain stimulation/modulation technologies (invasive or noninvasive) for use in the treatment of psychiatric disorders, or in targeting specific domains of clinical functioning across psychiatric disorders, when appropriate (see RDOC). Devices capable of both recording and stimulating neural activity, with the ability for closed-loop control are also of interest (including synchronizing dense behavioral quantification with neural data); these devices should be able to demonstrate clear capability to record oscillations of interest to mental health applications. Devices can target specific age ranges, including vulnerable populations (pediatric, geriatric). Note: Animal studies to assess “efficacy” must follow NIMH criteria. Please contact NIMH staff above to ensure your project fits NIMH priorities, prior to application submission.
Office of Behavioral and Social Sciences Research (OBSSR)
Contact: Dana Greene (formerly Schloesser), Ph.D.
National Institute of Neurological Disorders and Stroke (NINDS)
Contact: Nick Langhals, Ph.D.; Emily Caporello, Ph.D.
NINDS will support translational device applications relevant to its mission.
National Institute on Aging (NIA)
Contact: Kristina McLinden, Ph.D.; Yuan Luo, Ph.D.
NIA, as the primary federal agency for aging and Alzheimer’s Disease and related dementias (AD/ADRD) research, supports the development and application of innovative technology for early diagnosis and treatment of age-related disorders and AD/ADRD.
National Institute on Alcohol Abuse and Alcoholism (NIAAA)
Contact: Elizabeth Powell, Ph.D.
NIAAA’s mission is to generate and disseminate fundamental knowledge about the adverse effects of alcohol on health and well-being and to apply that knowledge to improve diagnosis, prevention, and treatment of alcohol-related problems, including alcohol use disorder (AUD), across the life span. NIAAA is interested in wearable devices that can monitor blood alcohol concentration in real time, non-invasive methods for the treatment of fetal alcohol spectrum disorders (FASD) in children and adults, and in the treatment of AUD.
National Institute on Drug Abuse (NIDA)
Contact: Leonardo Angelone, Ph.D.; Dan Kostov, Ph.D.
NIDA will support applications aiming to develop novel medical devices intended for use in the diagnosis of, or in the cure, mitigation, treatment, or prevention of Substance Use Disorder (e.g., Opioid Use Disorder, Stimulant Use Disorder). See the NIDA Mission for additional details.
Common Data Element Usage
Please note that many Blueprint ICs expect or strongly encourage the collection of Common Data Elements (CDEs) for the research supported by their IC. Applicants to BP MedTech NOFOs are encouraged to contact the POC for relevant ICs for more information on IC-specific data sharing expectations and requirements
BP MedTech Resources and Support Services
BP MedTech will support grantees by providing commercialization, verification, and validation resources. Resources anticipated to be provided by BP MedTech-funded Incubator Hubs are listed first below. All other resources will be provided through the three awarded BP MedTech resource contracts.
Available resources are anticipated to include:
Design, Prototyping, and Risk Analysis
- Electronics Manufacturing
- Prototype Development
- Design Optimization and Risk
- Computational Modeling
Bench and Safety Testing
- Electrical Safety
- Electromagnetic Compatibility
- MR Testing
- Software
- Cybersecurity
- Shelf-life Testing
Biocompatibility and Animal Studies
- Preclinical Studies – Animal Testing (non-GLP)
- Cadaver Testing
- Biocompatibility and Animal Studies Advising
- Biocompatibility Testing to ISO10993 standards
- Materials characterization and analytical chemistry
- Sterilization testing/validation
- Preclinical Studies – Animal Testing (GLP)
Clinical Support
- Neuroethics
- Clinical Support Advising
- Clinical Trials
- Protocol development and study preparation
- Study implementation, including protocol implementation and oversight
- Regulatory and compliance documentation, including audit support
- Biostatistics
- Clinical study analysis, close-out, and final study reporting
- Data Management
- Database support (if applicable)
- Imaging support
- Investigational device management and specimen collection
Business Development
- Entrepreneurship
- Business Development
- Market / User Research
- Commercialization
- External Oversight Committee
- Public-Private Partnerships – CRA, MTA
Regulatory, Compliance, and Quality System
- Regulatory Advising
- QMS – Quality Management System – setup and audits
- GMP – Good Manufacturing Practice – setup and audits
- Compliance
Legal
- Intellectual Property consulting; includes drafting patent applications, patent prosecution (Office Action responses), freedom to operate analyses, patentability reports, and landscape analyses
Note: filing fees to the USPTO and WIPO/PCT are not allowable - Corporate structuring and governance; includes counsel on incorporation and strategic partnerships
- Licensing
BPMT External Oversight Committee
The Blueprint MedTech (BPMT) External Oversight Committee (EOC) is an external advisory group of industry, clinical, regulatory, non-profit, insurance and academic experts in device development and translation. The EOC provide independent advice to the NIH program staff and scientific and strategic inputs for its current projects. They have executive-level expertise in therapeutic and diagnostic medical devices development experience, with experience in portfolio-level decision making in both academia and industry.
For more information, please contact Blueprint MedTech
Amy Kruse, PhD
Sanjay N. Misra, MD, MBA
Clare Padgett, MBA
Katrina Gwinn, MD, FAAN
Rhonda Robinson Beale, MD
John Donoghue, PhD
Khara Ramos, PhD
Contact Blueprint MedTech Staff
Leonardo Angelone, Ph.D. (NIDA lead)
Nick Langhals, Ph.D. (NINDS lead)
Michael Wolfson, Ph.D. (NIBIB lead)
Eunyoung Kim, Ph.D. (NIMH lead)
Stacie Gutowski, Ph.D. (NIBIB)
Yordan (Dan) Kostov, Ph.D. (NIDA)
Sarah Robinson Schwartz, Ph.D. (NINDS)
Questions? Contact us at: Blueprint MedTech
