
Alexis N. Simpkins, M.D., Ph.D.
Clinical Assistant Professor
Stroke Division, Department of Neurology
University of Florida
Integrating scientific and clinical practice
My interest in research was sparked as an undergrad during a summer program at the Medical College of Georgia. I worked on a project in Dr. Susan Fagan’s lab to develop an assay that would allow blood to be accurately measured in an animal model of stroke, which could be used to assess the effectiveness of drugs being studied in the pre-clinical setting. Once I learned how medical research could have such a broad impact on patient care, I could not envision a career without incorporating both clinical care and medical research. This experience set me on my path to becoming a vascular neurologist, a path that was solidified when my father had a stroke that affected his speech. Seeing first-hand how the ramifications of a stroke can drastically change a person’s life greatly affirmed my choice to become a neurologist.
I have been supported by NIH funding throughout my training career. After undergrad, I completed my MD/PhD at the Medical College of Georgia. During graduate school, I was funded on an NIH T32 institutional training grant and an individual F31 fellowship award. The fellowship taught me how to write grants, present scientific data, critique published articles, and how to maintain collaborative working relationships with peers and other labs. My graduate work in Dr. John Imig’s lab focused on the protective effects of soluble epoxide hydrolase inhibitors in cerebral ischemia and vascular remodeling in the brain; a natural complement to my undergraduate focus on preclinical assays.
After my MD/PhD and internship, I completed a neurology residency at the Johns Hopkins University. During residency, I was able to continue to do research through an NINDS R25 Research Education Program for Residents and Fellows in Neurology and Neurosurgery. In addition to the protected time for research, the R25 funding provided invaluable mentoring and career advice. My goal during the R25 was to find a therapeutic agent that could potentiate recovery and be an effective medication for secondary stroke prevention. I worked in Dr. Raymond Koehler’s lab in collaboration with Dr. Sujatha Kannan on the role of eicosanoids and inflammation and microglial activation in a slice culture model of cerebral ischemia. Even though my results were promising, I recognized the limitations in translating pre-clinical research in animal models and in vitro studies to the bedside.
To address this gap, I decided to add two years of research to my one year ACGME clinical vascular neurology fellowship at the National Institutes of Health. My research at NIH with Drs. Richard Leigh, John Hallenbeck, and Lawrence Latour focused on imaging and blood biomarkers in human acute stroke, and was really only possible to conduct with the resources and facilities at NIH. I was able to spend two additional years as a fellow in part because of the NIH Intramural Loan Repayment Program, which paid back some of my student loans while I was still in training. These two additional years of research gave me a large chunk of protected research time, which can be hard for early career physicians to obtain. It allowed me to get the preliminary data and publications (one of which was awarded the 2016 Progress and Innovation Award by the journal Stroke) that I will need to prepare for submitting a grant to support my research early in my faculty appointment.
During my fellowship, I was also accepted into the NINDS-funded R25 TRANSCENDS program, which is a mentorship program through the American Academy of Neurology designed to help young investigators of diverse background successfully apply for NIH career development (K) awards. This program and the R25 I participated in during residency have been invaluable in providing mentorship and helping me select a faculty position that would fit my research and clinical priorities. In July 2017, I started a faculty position at the University of Florida, and will soon begin preparing my career development (K) award application. I plan to use the time, funding, and mentorship during the K award period to further develop into an independent researcher.
As a clinician scientist, I have a dual perspective that serves me well in both the clinic and at the bench. My medical training has allowed me to ask research questions driven by the problems I see my patients encounter and the limitations of treatment options that I can offer to patients. For example, patients can find it difficult to juggle taking multiple medications (such as separate drugs for hypertension, cholesterol, and diabetes). This can make medicine compliance an issue. With that in mind, the class of drugs that I was researching in graduate school had pleiotropic effects that could potentially reduce risk of cardiovascular disease and stroke, and thus limit the number of medications that would have to be prescribed, easing the burden on patients.
In turn, my scientific training has provided me with critical thinking skills and the ability to separate meaningful information from the noise. It helped me gain the skills to review the clinical literature and decide what should become part of my clinical practice. It has also let me have a broader impact on patient care than I can achieve by treating individual patients. Although my education has been long, it has provided me with a unique perspective and opportunity to make a difference.
- As told to Lauren Ullrich
Current Research
At times, it is not clear which patients may benefit from an acute treatment for ischemic stroke and which patients may be harmed by interventions aimed at restoring blood flow to ischemic brain tissue. Determining early factors that can predict a patient’s clinical outcome after stroke would allow clinicians to better select patients most likely to benefit from therapy and while protecting those at risk of harm from interventions. MRI brain imaging can be used to help guide therapy acutely since it is non-invasive and quick way to evaluate the characteristics of the stroke and guide clinical decisions on acute therapy. Interestingly, the appearance of ischemic stroke on brain MRI acquired very rapidly after acute stroke onset may not always predict if the patients will do well or poorly with acute intervention. This suggests that there are other factors occurring during acute stroke that are not captured by imaging alone. To better assess what other factors are ongoing at the time of an acute ischemic stroke, my lab is also incorporating biomarkers in blood. We have implemented a pilot study that serially acquires advanced MRI and next generation sequencing of RNA in the whole blood during the very early phases of ischemic stroke. By combining both the blood analysis and the brain imaging, we hope to be able to determine key biological contributors to patients’ response to therapy.
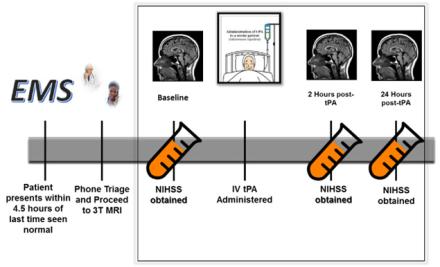
MRI brain imaging can be used to help guide therapy acutely since it is non-invasive and quick way to evaluate the characteristics of the stroke and guide clinical decisions on acute therapy.
