Director's Overview
The National Institute of Neurological Disorders and Stroke (NINDS) supports research that advances the diagnosis, prevention, and treatment of neurological disorders, that is, diseases of the brain, spinal cord, and neuromuscular system. Basic research to understand the brain in health and disease, which is the wellspring of both public and private sector progress, is at the core of this mission.
Chronic pain, dementias, stroke, traumatic brain injury (TBI), epilepsy, Parkinson’s disease, multiple sclerosis, cerebral palsy, brain tumors, and other common neurological disorders affect millions of Americans of all ages. Hundreds of rare diseases collectively add to the enormous impact. Increasing the urgency for progress, brain disorders are rising as the population ages, the high prevalence of poorly controlled chronic pain drives the opioid crisis, and unacceptable health disparities persist despite progress against many neurological disorders. Furthermore, neurological complications of COVID-19 are common, and there is considerable concern, but much uncertainty, about long-term neurological disability.
The multiplicity of neurological diseases and the brain’s complexity and sensitivity to disruption present formidable challenges to medical science. Despite these obstacles, since Congress established NINDS, research has produced remarkable progress in diagnosis, treatment, and prevention of neurological disorders. Advances in brain imaging, genetic testing, and other tools augment clinical diagnosis, often eliminating the years’ long diagnostic odysseys that families endured in the past. Moreover, research has brought effective treatment options when before there were none—more than a dozen drugs reduce symptoms and slow the course of multiple sclerosis; treatments enable many people with Parkinson’s disease to go about their lives for many years; and, similarly, physicians can offer better therapeutics for epilepsy, migraine, dystonia, and other neurological diseases. In the last few years, the first gene-targeted therapies have arrived for a few rare genetic disorders, with promising treatments for several others now in advanced testing. Deep brain stimulation (DBS) and other device therapies are under development for several diseases, building on successes for essential tremor, Parkinson’s disease, and epilepsy. Combinations of devices, drugs, and rehabilitation, together with extraordinary persistence, have enabled a few people paralyzed by spinal cord injuries to stand and take their first steps. For stroke, data from the Centers for Disease Control and Prevention (CDC) show that the age adjusted death rate from stroke has declined by seventy percent over fifty years, saving millions of lives1. This demonstrates the cumulative impact of breakthroughs in emergency treatments and continuous progress in prevention over many years.
NINDS alone is not responsible for these advances, but the Institute’s research has been pivotal, catalyzing progress across the public and private research landscape. Foremost among the Institute’s contributions is support for basic research—every advance described above depended on fundamental research to understand the healthy brain and what goes wrong in disease. Among the many other ways that NINDS spurs progress, laboratory proof-of-concept studies “de-risk” innovative therapeutic development strategies with long time horizons or a high risk of failure to attract private sector investment, as with recent breakthroughs in gene targeted therapies. Similarly, development of biomarkers and clinical outcome measures is often essential, as with the recent major advances in stroke treatment with clot retrieval devices, drugs for multiple sclerosis, and other innovations. Over forty years the NINDS Epilepsy Therapy Screening Program has provided standardized animal testing data, encouraging industry development of eleven drugs that are now on the market, and an NINDS program begun fifty years ago pioneered the now burgeoning field of therapeutic devices that interface with the nervous system. NINDS also stimulates progress via natural history of disease studies that guide clinical trials, epidemiological studies that identify modifiable risk factors, support for clinical trials on treatment and prevention, and development and dissemination of research resources and reagents. Perhaps most importantly for long term progress, NINDS supports training and career development for the nation’s neuroscience research workforce.
Although neurological disorders present many longstanding challenges, emerging public health issues also demand NINDS attention. NINDS investment in foundational basic research, workforce training, research resources, data sharing, collaboration, and experimental rigor, as well as flexible, efficient, and effective programs on biomarkers, preclinical therapy development, and clinical trials position the Institute to respond as new challenges arise. In the National Alzheimer’s Project Act (NAPA) and through targeted appropriations, Congress recognized the present and future public health impact of not only Alzheimer’s disease (AD), but also Alzheimer’s disease-related dementias (ADRDs). NINDS, working with the National Institute on Aging (NIA), leads an extensive program of NIH research on the ADRDs, which include vascular contributions to cognitive impairment and dementia (VCID), frontotemporal degeneration (FTD), Lewy body dementia (LBD), and mixed dementias. As the leading supporter of pain research, NINDS coordinates NIH Helping End Addiction Long-TermSM (HEAL) initiative programs, also supported with targeted appropriations, that are developing non-addictive pain treatments which displace addictive drugs or are effective when opioids are not. NIH is accelerating the development of new pain treatments by increasing consultation with experts in clinical pain management and drug development and by leveraging established preclinical drug development programs and resources within NINDS and NIH’s National Center for Advancing Translational Sciences (NCATS).
Most recently, NINDS has responded to the COVID-19 pandemic, focusing attention on the neurological complications of infection, whether caused directly by virus or indirectly by the body’s response. Symptoms, such as fatigue, headache, pain, confusion, cognitive slowing, or dizziness, are common, both in the acute illness and in those who remain unwell weeks or months later. In the acute phase, severe problems including stroke, delirium, encephalitis, seizures, and, possibly, direct effects on brain respiratory control circuits may also occur. There are also reports of rare but debilitating cases of acute necrotizing hemorrhagic encephalopathy, transverse myelitis, Guillain Barre syndrome, and multi-system inflammatory syndrome in children. NINDS research on COVID-19 has included rapid supplements to existing grants, development of a database on neurological symptoms observed in patients, clinical studies in the Intramural Research Program, and clinical trials to assess potential treatments and address stroke in COVID patients with the National Heart Lung and Blood Institute (NHLBI). NINDS also is working with NHLBI, National Institute of Allergy and Infectious Disease (NIAID), and other NIH Institutes and Centers to launch research to better understand and treat those persons with long term disabling symptoms after COVID infection.
NINDS has long established two priorities that are vital to Institute’s mission across all programs: workforce development and health disparities. The extraordinary challenges of neurological disorders require engaging the nation’s full talent pool. To that purpose, the NINDS Office of Programs to Enhance Neuroscience Development (OPEN) coordinates an array of programs at the individual, institutional, and community level, both within NINDS and jointly with other parts of the NIH. Among these, research supplements to promote support to scientists from the high school to career development level via existing research grants; Institutional grants include Summer Research Experience Programs and the Blueprint ENDURE (Enhancing Neuroscience Development through Undergraduate Research Education) program; and the D-SPAN (Specialized Predoctoral to Postdoctoral Advancement in Neuroscience) Mentored Career Development Awards and the Faculty Development Award provide support at critical career transition points. NINDS also fosters networks for mentorship and considers whether investigators bring diverse perspectives as one factor in select pay decisions. Similarly, health disparities are a priority throughout all Institute programs. Despite progress across all population groups, substantial disparities persist for minority populations for disorders that afflict young children, adults, and older Americans. Although the Institute has supported groundbreaking research, especially in stroke, that has characterized disparities and identified risk factors, the NINDS must now push forward to eliminate those disparities. Development of an NINDS strategic plan for health disparities is now underway to guide the Institute forward.
The imperative for progress is evident every day to people affected by neurological disorders, their families, and the physicians who treat them. Although the unmet needs are immense, so are the opportunities for progress. The CDC reports that, despite considerable progress, more than 795,000 people in the United States still suffer strokes each year, with about 150,000 deaths and 7 million survivors, many with major disabilities 2. However, the recent improvements in stroke emergency treatment expand the time window for effective intervention, opening opportunities to develop effective adjunct therapies that protect the brain. In dementia, VCID and Alzheimer’s disease are so intertwined that most elderly people with dementia have a combination of the two, and the overall numbers are rising as the population ages. But emerging evidence suggests that interventions to prevent stroke, also a brain vascular disorder, may help prevent dementia, with recent clinical trial results adding to the evidence base. No existing therapies are effective for about a third of people with epilepsy, prevention of epilepsy is still elusive, and all anti-seizure drugs carry troublesome side effects. NINDS programs are reinvigorating their focus on drug resistant epilepsy and on preventing the development of epilepsy, building on advances in basic research. For hundreds of rare genetic neurological disorders, many of which affect infants and children, we know the gene mutations responsible, but have no disease modifying therapy. The new NINDS Ultra-Rare Gene Therapy (URGenT) Network will take advantage of recent progress in gene targeting technologies to rapidly develop tailored therapeutic interventions using precision medicine platforms for the treatment serious, life-threatening ultra-rare diseases and blaze a wider path for genomic therapy in brain disorders. Likewise, basic research on the mechanisms that underlie chronic pain has identified many new targets for developing non-addictive drugs and devices, which NIH HEAL Initiative programs are aggressively targeting. Although the challenges of neurological diseases are daunting, opportunities for progress abound.
Looking forward long-term, the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative, supported by the 21st Century Cures Act and directed appropriations, is shaping a future in which we understand how dysfunctional brain circuits cause brain disorders and can precisely correct the problems. How information is processed in complex human brain circuits is largely a mystery, and this limits the effectiveness of diagnostic and therapeutic strategies to improve brain circuitry affected by neuro/mental/substance abuse disorders. Although the BRAIN Initiative’s focus is on developing the tools to map brain circuits and monitor and modulate circuit activity in the normal brain, the technologies and foundational knowledge provide powerful means to combat brain diseases. The extent to which advances from BRAIN are already launching new approaches to study and treat neurological, mental health, and substance abuse disorders is encouraging. Based on the BRAIN Cell Census program, investigators are determining which specific brain cell types are affected by dementia, autism, and many other diseases. BRAIN technologies also offer the promise of targeting treatments precisely to these cells, as well as devices for decoding of speech directly from brain activity, restoring vision, and self-adjusting “closed-loop” electrical stimulation therapies, among many other possibilities. Given the power of these new technologies, the BRAIN Initiative has also integrated thought and research into ethics that guide their use. The BRAIN Initiative is providing a major thrust toward realizing the Institute’s guiding vision: a world that is free from the burden of neurological disorders.
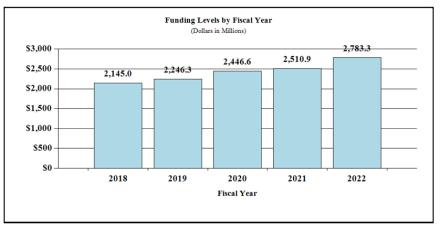
Overall budget policy: The FY 2022 President’s Budget request for NINDS is $2,783.3 million, an increase of $272.4 million or 10.8 percent compared to the FY 2021 Enacted level. The NINDS request includes 21st Century Cures Act funding of $76.0 million for the BRAIN Initiative, an increase of $26.0 million from the FY 2021 Enacted level. The proposed increase in FY 2022 also includes an increase of $135.1 million for the HEAL Initiative accompanied by a $43.0 million increase in other NINDS opioid and pain research.
_______________________________________________
1Vital Signs: Recent Trends in Stroke Death Rates — United States, 2000–2015
IC Fact Sheet
View IC Fact Sheet(pdf, 522 KB)
Major Changes in the Fiscal Year 2022 President’s Budget Request
Major changes by budget mechanism and/or budget activity detail are briefly described below. Note that there may be overlap between budget mechanism and activity detail, and these highlights will not sum to the total change for the FY 2022 President’s Budget request for NINDS, which is $2,783.3 million, an increase of $272.4 million from the FY 2021 Enacted level. Within the President’s Budget request level, NINDS will pursue its highest research priorities through strategic investments and careful stewardship of appropriated funds.
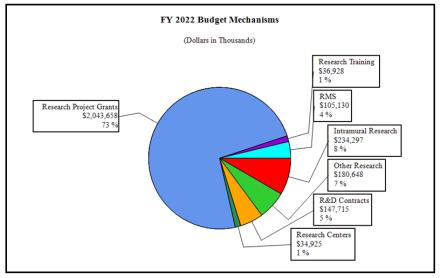
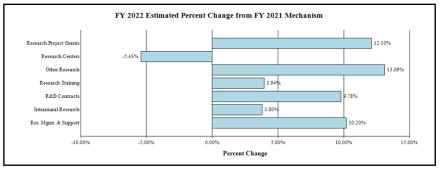
Research Project Grants (RPGs) (+$220.6 million; total $2,043.7 million):
The NINDS budget reflects an increase of $215.8 million in the Research Project Grants portfolio, including SBIR/STTR awards. Competing RPGs are expected to increase by 87 grants in FY 2022 compared to the FY 2021 enacted level of awards.
Other Research (+$20.9 million; total $180.6 million):
The Other Research mechanism reflects an increase due to the increase in Other Research grants funded from HEAL Initiative funding. NINDS plans to increase its regular portfolio of Other Research as well.
Research Management and Support (+$9.7 million; total $105.1 million):
The NINDS budget reflects an increase of $9.7 million in Research Management and Support to provide additional staff and other support in recognition of recent increases in the NINDS grant portfolio.
Budget Mechanism - Total 1,2
(Dollars in Thousands)
| MECHANISM | FY 2020 Final3 | FY 2021 Enacted | FY 2022 President's Budget | FY 2022 +/- FY 2021 Enacted | ||||
|---|---|---|---|---|---|---|---|---|
| No. | Amount | No. | Amount | No. | Amount | No. | Amount | |
| Research Projects: | ||||||||
| Competing: | ||||||||
Research Centers: | ||||||||
Other Research: | ||||||||
Ruth L. Kirschstein Training Awards: | FTTPs | FTTPs | FTTPs | |||||
| Noncompeting | 2,092 | $1,100,881 | 2,289 | $1,205,975 | 2,473 | $1,291,458 | 184 | $85,482 |
| Administrative Supplements | (135) | 14,575 | (170) | 22,986 | (95) | 17,986 | (-75) | -5,000 |
| Renewal | 104 | 61,931 | 106 | 65,526 | 107 | 63,636 | 1 | 1,110 |
| New | 899 | 500,852 | 800 | 440,052 | 888 | 575,372 | 88 | 135,320 |
| Supplements | 10 | 6,543 | 8 | 5,650 | 6 | 4,500 | -2 | -1,150 |
| Subtotal, Competing | 1,013 | $569,327 | 914 | $508,228 | 1,001 | $643,508 | 87 | $135,280 |
| Subtotal, RPGs | 3,105 | $1,684,782 | 3,203 | $1,737,190 | 3,474 | $1,952,952 | 271 | $215,762 |
| SBIR/STTR | 103 | 77,142 | 110 | 85,838 | 114 | 90,706 | 4 | 4,868 |
| Research Project Grants | 3,208 | $1,761,925 | 3,313 | $1,823,028 | 3,588 | $2,043,658 | 275 | $220,630 |
| Specialized/Comprehensive | 31 | $34,077 | 33 | $36,694 | 32 | $34,688 | -1 | -$2,006 |
| Clinical Research | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Biotechnology | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Comparative Medicine | 0 | 0 | 0 | 237 | 0 | 237 | 0 | 0 |
| Research Centers in Minority Institutions | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| Research Centers | 31 | $34,177 | 33 | $36,931 | 32 | $34,925 | -1 | -$2,006 |
| Research Careers | 239 | $44,337 | $261 | $48,497 | 309 | $50,831 | 48 | -$2,334 |
| Cancer Education | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cooperative Clinical Research | 0 | 4,508 | 1 | 1,852 | 1 | 2,000 | 0 | 148 |
| Biomedical Research Support | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Minority Biomedical Research Support | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 229 | 107,121 | 231 | 109,391 | 296 | 127,816 | 65 | 18,425 |
| Other Research | 469 | $155,966 | 493 | $159,740 | 606 | $180,648 | 113 | $20,907 |
| Total Research Grants | 3,708 | $1,952,067 | 3,839 | $2,019,699 | 4,226 | $2,259,230 | 387 | $239,532 |
| Individual Awards | 378 | 17,766 | 383 | $17,952 | 387 | $18,469 | 4 | $516 |
| Institutional Awards | 305 | 17,137 | 323 | 17,577 | 327 | 18,459 | 4 | 882 |
| Total Research Training | 683 | $34,904 | 706 | $35,529 | 714 | $36,928 | 8 | $1,399 |
Research & Development Contracts | 117 | $148,947 | 125 | $134,560 | 132 | $147,715 | 7 | $13,155 |
| SBIR/STTR (non-add) | (3) | (1,048) | (5) | (1,093) | (3) | (1,126) | (-2) | (33) |
| Intramural Research | 300 | 219,606 | 314 | $225,723 | 351 | 234,297 | 37 | 8,574 |
| Research Management and Support | 225 | 91,054 | 235 | 95,402 | 256 | 105,130 | 21 | 9,728 |
| Res. Management & Support (SBIR Admin) (non-add) | (0) | (460) | (0) | (460) | (0) | (681) | (0) | (221) |
| Construction | 0 | 0 | 0 | 0 | ||||
| Buildings and Facilities | 0 | 0 | 0 | 0 | ||||
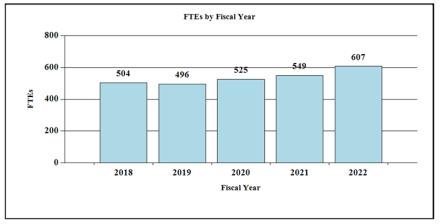
| Total, NINDS | 525 | $2,446,577 | 549 | $2,510,913 | 607 | $2,783,300 | 58 | $272,387 |
1 All items in italics and brackets are non-add entries.
2 Of which $70.0 million in FY 2019, $70.0 million in FY 2020, and $50.0 million in FY 2021, and $76.0 million in FY 2022 is derived by transfer from the NIH Innovation Account under the 21st Century Cures Act.
3 Includes $6.1 million of 21st Century Cures Act funding not obligated in FY 2020 and carried over into FY 2021.
Appropriations Language
For carrying out section 301 and title IV of the PHS Act with respect to neurological disorders and stroke, [$2,463,393,000]$2,219,298,000.
NIH INNOVATION ACCOUNT, CURES ACT (INCLUDING TRANSFER OF FUNDS)
For necessary expenses to carry out the purposes described in section 1001(b)(4) of the 21st Century Cures Act, in addition to amounts available for such purposes in the appropriations provided to the NIH in this Act, [$404,000,000]$496,000,000, to remain available until expended: Provided, That such amounts are appropriated pursuant to section 1001(b)(3) of such Act, are to be derived from amounts transferred under section 1001(b)(2)(A) of such Act, and may be transferred by the Director of the National Institutes of Health to other accounts of the National Institutes of Health solely for the purposes provided in such Act: Provided further, That upon a determination by the Director that funds transferred pursuant to the previous proviso are not necessary for the purposes provided, such amounts may be transferred back to the Account: Provided further, That the transfer authority provided under this heading is in addition to any other transfer authority provided by law. Department of Health and Human Services Appropriations Act, 2020.
Summary of Changes
(Dollars in Thousands)
| F Y 2021 Enacted | $2,510,913 |
|---|---|
| F Y 2022 President's Budget | $2,783,300 |
| Net change | $272,387 |
| FY 2021 Enacted | FY 2022 President's Budget | Built-in Change from FY 2021 Enacted | ||||
|---|---|---|---|---|---|---|
| CHANGES | FTE's | Budget Authority | FTEs | Budget Authority | FTEs | Budget Authority |
| A. Built-in: 1. Intramural research: | ||||||
| 2. Research Management and Support: | ||||||
| a. Annualization of January 2021 pay increase & benefits | $59,840 | $67,559 | $162 | |||
| b. January FY 2022 pay increase & benefits | 59,840 | 67,559 | 1,641 | |||
| c. Paid days adjustment | 59,840 | 67,559 | 0 | |||
| d. Differences attributable to change in FTE | 59,840 | 67,559 | 7,380 | |||
| e. Payment for centrally furnished services | 36,092 | 37,897 | 1,805 | |||
| f. Cost of laboratory supplies, materials, other expenses, and non-recurring costs | 129,790 | 128,841 | 3,653 | |||
| Subtotal | $14,640 | |||||
| a. Annualization of January 2020 pay increase & benefits | $42,208 | $45,299 | $112 | |||
| b. January FY 2021 pay increase & benefits | 42,208 | 45,299 | 1,170 | |||
| c. Paid days adjustment | 42,208 | 45,299 | 0 | |||
| d. Differences attributable to change in FTE | 42,208 | 45,299 | 3,899 | |||
| e. Payment for centrally furnished services | 7,457 | 7,830 | 373 | |||
| f. Cost of laboratory supplies, materials, other expenses, and non-recurring costs | 45,738 | 52,001 | 1,188 | |||
| Subtotal | $6,742 | |||||
| Subtotal, Built-in | $21,382 | |||||
| FY 2021 Enacted | FY 2022 President's Budget | Program Change from FY 2021 Enacted | ||||
|---|---|---|---|---|---|---|
| CHANGES | No. | Amount | No. | Amount | No. | Amount |
| B. Program: | ||||||
| 1. Research Project Grants: | ||||||
| a. Noncompeting | 2,688 | $1,228,961 | 2,473 | $1,309,444 | 184 | $80,482 |
| b. Competing | 914 | 508,228 | 1,001 | 643,508 | 87 | 135,280 |
| c. SBIR/STTR | 110 | 85,838 | 114 | 90,706 | 4 | 4,868 |
| Subtotal, RPGs | 3,313 | $1,823,028 | 3,588 | $2,043,658 | 275 | $220,630 |
| 2. Research Centers | 33 | $36,931 | 32 | 34,925 | -1 | $2,006 |
| 3. Other Research | 493 | 159,740 | 606 | 180,648 | 113 | 20,907 |
| 4. Research Training | 706 | 35,529 | 714 | 36,928 | 8 | 1,399 |
| 5. Research and Development Contracts | 125 | 134,560 | 132 | 147,715 | 7 | 13,155 |
| Subtotal, Extramural | $2,189,788 | $2,443,873 | $254,085 | |||
| FTEs | FTEs | FTEs | ||||
| 6. Intramural Research | 314 | $225,723 | 351 | $234,297 | 37 | -$6,066 |
| 7. Research Management and Support | 235 | 95,402 | 256 | 105,130 | 21 | 2,986 |
| 8. Construction | 0 | 0 | 0 | |||
| 9. Buildings and Facilities | 0 | 0 | 0 | |||
| Subtotal, Program | 549 | $2,510,913 | 607 | $2,783,300 | 58 | $251,005 |
| Total Changes | $272,387 | |||||
Fiscal Year 2022 Budget Graphs
History of Budget Authority and FTE's:
Distribution of Mechanism:
Change by Selected Mechanism:
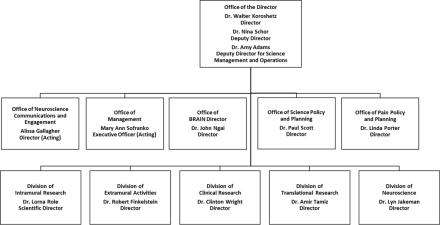
Organization Chart
Budget Authority by Activity1
(Dollars in Thousands)
| FY 2020 Final | FY 2021 Enacted | FY 2022 President's Budget | FY 2022 +/- FY 2021 Enacted | |||||
|---|---|---|---|---|---|---|---|---|
| Extramural Research Detail: | FTEs | Amount | FTEs | Amount | FTEs | Amount | FTEs | Amount |
| Division of Neuroscience | $1,481,227 | $1,521,379 | $1,617,666 | $96,287 | ||||
| Division of Clinical Research | 157,844 | 164,235 | 172,425 | 8,190 | ||||
| Division of Translational Research | 156,703 | 160,178 | 171,175 | 10,997 | ||||
| Division of Extramural Activities | 99,631 | 103,528 | 108,764 | 5,236 | ||||
| Opioid Research2 | 240,513 | 240,468 | 373,843 | 133,375 | ||||
| Subtotal, Extramural | $2,135,918 | $2,189,788 | $2,443,873 | $254,085 | ||||
Intramural Research | 300 | $219,606 | 314 | $225,723 | 351 | $234,297 | 37 | $8,574 |
| Research Management & Support | 225 | $91,054 | 235 | $95,402 | 256 | $105,130 | 21 | $9,728 |
| TOTAL | 525 | $2,446,577 | 549 | $2,510,913 | 607 | $2,783,300 | 58 | $272,387 |
1 Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
2 Total for Opioid/Pain Research including IR and RMS is (in thousands) $266,321 in FY 2020, $270,295 in FY 2021, and $405,443 in FY 2022.
Justification of Budget Request
National Institute of Neurological Disorders and Stroke
Authorizing Legislation: Section 301 and Title IV of the Public Health Service Act, as amended.
Budget Authority (BA):
| FY 2020 Final | FY 2021 Enacted | FY 2022 President's Budget | FY 2022 +/- FY 2021 | |
|---|---|---|---|---|
| BA | $2,446,577,000 | $2,510,913,000 | $2,783,300,00 | +$272,387,000 |
| FTE | 525 | 549 | 607 | 58 |
Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
Program Descriptions
Division of Neuroscience (DON)
As the largest part of the NINDS extramural program, the Division of Neuroscience supports research on the normal brain, spinal cord, and nerves of the body; the mechanisms and consequences of neurological injury and disease; and the early development of diagnostics and treatments. Investigator-initiated research is the foundation for the DON portfolio. In addition, targeted programs focus on specific research topics and public health priorities that are not fully addressed through investigator-initiated research. DON programs also support research resources, core facilities, and scientific conferences. Examples of program areas include:
- Basic neuroscience research: Gaps in understanding normal nervous system development and function can form roadblocks to addressing neurological disorders. Research to fill those gaps enables unanticipated breakthroughs and is a critical part of the NINDS mission that is unlikely to receive sustained support from the private sector. Because basic research benefits from the freedom to follow scientific opportunity, investigator-initiated research makes up the majority of the NINDS basic research portfolio. NINDS also encourages fundamental basic research through a targeted initiative with set-aside funding. In neuroscience as in other fields, new research tools drive new discoveries. NINDS supports the development and application of tools and technologies for neuroscience research, and this is also a major focus of the NIH BRAIN initiative, in which NINDS is a leading partner.
- Neurodegeneration: NINDS leads NIH support for research on many neurodegenerative diseases. For example, programs for Parkinson’s disease (PD) include the Morris K. Udall Centers of Excellence for Parkinson’s Disease Research supporting collaborative studies on the causes of PD; the Parkinson’s Disease Biomarkers Program (PDBP) to find biomarkers for PD and Lewy Body dementia (LBD); and the Accelerating Medicines Partnership for Parkinson’s Disease, which leverages patient cohorts and resources from PDBP and other programs for large-scale analyses to identify biomarkers and new targets for therapies. As part of the National Plan to Address Alzheimer’s Disease, NINDS leads research on Alzheimer’s Disease Related Dementias (ADRD), which include LBD, frontotemporal dementia, vascular contributions to cognitive impairment and dementia, and mixed etiology dementias. NINDS receives co-funding for ADRD research from the National Institute on Aging (NIA), which enables support for specific initiatives and an extended payline for meritorious proposals in this area.
- Rare diseases: Many rare diseases affect the nervous system, and research on these disorders often yields insights into more common diseases with shared mechanisms. NINDS supports investigator-initiated and targeted research on rare diseases and is a partner in the NIH Rare Diseases Clinical Research Network, funding consortia for lysosomal disorders, mitochondrial diseases, dystonia, and others. NINDS has enabled new treatments for rare neurological disorders, including the first gene-based, disease-modifying therapies for spinal muscular atrophy and muscular dystrophy. An ongoing NINDS initiative aims to fill gaps in clinical trial readiness for rare neurological and neuro-muscular diseases with new therapies on the horizon. NINDS also is partnering with the NIH Common Fund to dramatically increase knowledge about amyotrophic lateral sclerosis (ALS), via the new Accelerating Leading-edge Science in ALS (ALS2) initiative.
- Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): Researchers do not yet understand the causes of ME/CFS, and diagnostic tests and treatments are not available. Along with the National Institute of Allergy and Infectious Diseases (NIAID) and other Institutes and Centers, NINDS supports ME/CFSnet, a network of collaborative research centers that aims to develop and identify new diagnostics, biomarkers, and ways to stratify patients into subgroups based on clinical presentation. ME/CFSnet research suggests that immune dysregulation and differences in immune cell metabolism contribute to ME/CFS. NINDS also supports NIH funding opportunities to stimulate ME/CFS research and will be a lead participant in NIH strategic planning for ME/CFS, that began in Fall 2020.
- Epilepsy: Since 2000, NINDS has worked with the scientific and patient communities to establish shared research priorities called the Benchmarks for Epilepsy Research, and in September 2020, NINDS began gathering broad input on updated Benchmarks to guide future research. The NINDS Centers without Walls program for epilepsy research was designed to address priorities outlined by the Benchmarks. The newest center investigates how specific gene variants cause epilepsy, which will inform precision diagnostics and treatments. Other centers have focused on genetic causes of epilepsy; sudden unexpected death in epilepsy; and strategies to prevent epilepsy after traumatic brain injury (TBI).
- Other neurological disorders: DON research includes studies on many more nervous system disorders and on mechanisms shared across diseases, with studies suggesting paths to new and improved treatments and diagnostics for conditions including hydrocephalus, spinal cord injury, TBI, and neuropathies, among others. Because neuroscience spans scientific disciplines, NINDS also works closely with other NIH Institutes in areas of complementary interest. For example, in addition to playing a leading role in the NIH HEAL InitiativeSM, NINDS is the lead NIH Institute for pain research and heads the NIH Pain Consortium, which includes 23 Institutes and Centers that support pain research. Other areas of collaboration include muscular dystrophies, brain tumor, neuroimmune conditions such as multiple sclerosis and nervous system infections, and developmental disorders such as cerebral palsy, autism, Fragile X Syndrome, and Down syndrome. In the largest genetic study to date on cerebral palsy, NINDS-funded investigators estimate that genetic causes account for up to 14 percent of cases. The results stand to inform new directions for research and treatment.
Budget Policy: The FY 2022 President’s Budget request for the Division of Neuroscience is $1,617.7 million, an increase of $96.3 million, or 6.3 percent, from the FY 2021 Enacted level.
Brain circuit dysfunction underlies the loss of memory and cognition in dementia, altered movement in Parkinson’s disease and dystonia, seizures in epilepsy, persistence of chronic pain, and other neurological disorders. However, research tools have not been powerful enough to answer fundamental questions about how brain circuits work and what goes wrong in disease, impeding progress. The trans-NIH BRAIN Initiative exploits advances from many scientific and engineering disciplines to develop and apply tools that overcome these limitations, enabling researchers to identify all cell types in the brain, monitor thousands of cells in real time, precisely modulate brain circuits, and map intricate connections among nerve cells.
In 2014, the report BRAIN 2025: A Scientific Vision provided a vision, operating principles, goals, and milestones for the Initiative. Teams across NIH manage the program, led by NINDS and NIMH and coordinated with complementary programs in other agencies and the private sector. In 2019, an external BRAIN 2.0 group found progress to be so remarkable to warrant investment in larger scale, transformative projects that might propel neuroscience far into the future. These new projects will create a comprehensive parts list of human brain cell types, tools to access and modulate specific brain cell types, and complete wiring diagrams of the mouse brain and long-range nerve pathways in primate (including human) brains.
To date, the BRAIN Initiative has focused on the normal brain, anticipating that this will ultimately provide tools and knowledge to combat human brain diseases. The extent to which avenues are opening for progress against human disease is encouraging. New tools in development reveal precisely which human brain cells are affected by diseases, with the potential to direct treatment to them. Other examples include new approaches to deliver drugs to specific targets in the brain, self-tune deep brain stimulation therapy, decode movement or speech commands directly from brain activity, and improve the precision of noninvasive imaging.
Research at the frontiers of neuroscience raises potential ethical issues, requiring thoughtful consideration for anticipating and navigating the challenges arising from new brain technologies. The Initiative’s Neuroethics Working Group provides leadership in this domain.
Division of Translational Research (DTR)
The NINDS Division of Translational Research leads extramural NINDS therapy development, through a suite of milestone-driven research programs and services designed to support all development stages for drugs, devices, and biologic therapies, from preclinical studies to first in human clinical trials. Translational research is failure-prone and poses risks for private sector investment. DTR programs help to remove these risks by advancing therapies to a point of readiness sufficient for industry interest, or in some cases, for testing in NINDS-funded clinical trials. In FY 2022, NINDS will support the following DTR programs.
- The Blueprint Neurotherapeutics Network (BPN), led by NINDS for the NIH Blueprint for Neuroscience Research, focuses on small molecule drug development and will expand in FY 2022 to include biologic therapies. The BPN has supported 30 projects across eight Institutes and Centers since 2011. Three BPN compounds are in Phase II clinical trials, and the BPN and the HEAL InitiativeSM supported the development of a non-narcotic analgesic for neuropathic pain that has received fast track designation from the FDA.
- The Innovation Grants to Nurture Initial Translational Efforts (IGNITE) program funds early-stage therapy development, such as validation of assays to evaluate proposed agents, demonstration that therapies have sufficient biological activity to warrant further investment, and development of model systems for early testing. IGNITE-supported therapies for epilepsy and Parkinson’s disease have advanced to the BPN for later stage studies.
- The Cooperative Research to Enable and Advance Translational Enterprises program (CREATE) supports the development of biologic therapies, including large biological molecules, cell therapies, and gene therapies. Many therapies in CREATE are based on antisense oligonucleotides (ASOs) designed to modify the expression of specific genes, reflecting the promise of such precision medicine approaches for neurological diseases. A recent project led to a clinical trial of an ASO to treat ALS. The program is also poised to advance other new approaches, such as genome editing, for therapeutic use.
- The Translational Neural Devices program supports therapeutic and diagnostic device development for disorders of the nervous system, from preclinical studies through early stage clinical trials. Recent advances include devices that restore respiratory muscle function in spinal cord injury and stimulate the vagus nerve to engage neuromodulatory circuits and improve motor and sensory function after stroke. NINDS also manages device-related programs in the NIH HEAL and BRAIN initiatives and will jointly manage a new NIH Blueprint MedTech program.
- The NINDS Biomarkers program supports the development and validation of biomarkers that can aid clinical trials and treatment decisions. Projects focus on, for example, diagnostic biomarkers for MS, Parkinson’s disease, Friedreich's ataxia, fibromyalgia, and an imaging method that could triage patients with stroke due to large vessel occlusion.NINDS also leads a biomarker program for pain conditions as part of the HEAL InitiativeSM.
- The Epilepsy Therapy Screening Program (ETSP) screens candidate compounds from academia and industry in standardized animal models and has contributed to 11 drugs approved for common and rare forms of epilepsy, most recently Epidiolex (cannabidiol) for seizures in Lennox-Gastaut and Dravet syndromes and Xcopri (cenobamate) for partial onset seizures. The program aims to advance new treatments for drug-resistant epilepsy, epilepsy syndromes, and disease prevention and modification. The ETSP served as a model for the Preclinical Screening Platform for Pain (PSPP), led by DTR for the HEAL InitiativeSM.
- The NIH Countermeasures Against Chemical Threats (CounterACT) program, funded through the NIH Office of the Director, develops medical countermeasures for toxic exposures after a chemical emergency. Several new drug candidates have moved to advanced development, including one FDA-approved for reducing seizures after nerve agent exposure. CounterACT is part of the NIH Biodefense Program led by NIAID.
- NINDS SBIR/STTR programs support research by small businesses to develop therapies, diagnostics, and research tools relevant to the NINDS mission. Several DTR programs, as well as the NIH BRAIN and HEAL initiatives, include SBIR/STTR funding opportunities.
Budget Policy: The FY 2022 President’s Budget request for the Division of Translational Research is $171.2 million, an increase of $11.0 million, or 6.9 percent, from the FY 2021 Enacted level.
NINDS is establishing the URGenT Network to support the development of state-of-the-art gene-based therapies for ultra-rare diseases, which affect one or less than one in 50,000 people. Around 7,000 known rare and ultra-rare diseases affect 30 million people in the US. About 45 percent of rare diseases are neurological, and 90 percent of rare childhood disorders have major neurological effects. Many are life-threatening, and few have FDA-approved treatments. Combined, ultra-rare diseases represent a large medical need, but the small number of people with each condition makes research difficult and limits incentives for industry investment.
Most rare diseases have a genetic origin, and causal mutations in ultra-rare diseases are unique to very few people, sometimes to a single patient. Successful gene-based therapies for some genetic diseases, including spinal muscular atrophy, have fueled promise for the rarest of diseases, and reports of custom-designed treatments for individual patients have gained public attention. Such efforts present challenges for safety and efficacy research, regulatory approval, and business processes built around larger patient populations.
URGenT is a late-stage preclinical therapy development program that aims to address challenges of gene-targeting technologies, de-risk these approaches for industry adoption, and coordinate their entry into clinical trials. The program will facilitate ways to standardize and share resources, data, and best practices across diseases to make therapy development for ultra-rare diseases more efficient and accessible. Project selection for URGenT will rely on strong scientific criteria, and only projects that meet milestones for progress will advance through successive phases of support, allowing NINDS resources to go to the most promising approaches.
In implementing URGenT, NINDS will engage with researchers, patient groups, government agencies involved in healthcare (including the FDA), and others on ways to reliably and sustainably deliver new therapeutics to patients with ultra-rare disorders.
Division of Clinical Research (DCR)
The Division of Clinical Research supports clinical trials infrastructure and large-scale clinical research, including early and advanced phase clinical trials, comparative effectiveness research, and epidemiological studies, for neurological conditions across the lifespan. To optimize clinical research, DCR enforces milestones for progress and provides resources to improve patient access and recruitment. DCR also develops clinical research initiatives and provides expertise in clinical trial design and statistics to researchers and the Institute. NINDS clinical networks and approaches to improve the efficiency and quality of clinical trials have served as models for other NIH programs, including the Early Phase Pain Investigation Clinical Network (EPPIC-Net), led by NINDS for the HEAL InitiativeSM. Recent DCR-supported studies have informed advances in understanding, treating, and preventing neurological disorders, in areas including stroke, epilepsy, Parkinson’s disease, MS, vascular risk factors for dementia, and others. NINDS also quickly deployed support for infrastructure and research to collect data on neurologic aspects of COVID-19. In FY 2022, major DCR programs will include:
- StrokeNet provides support and infrastructure for clinical trials on stroke treatment, prevention, and recovery and rehabilitation through 25 regional centers and more than 400 hospitals across the United States. Fourteen trials have been conducted or are ongoing through the network since it was established in 2014, generating results that inform clinical care for stroke patients. StrokeNet’s first trial for pediatric stroke aims to determine whether intense rehabilitation incorporating constraint-induced movement therapy improves upper extremity motor function in babies who have had a perinatal stroke.
- The Network for Excellence in Neuroscience Clinical Trials (NeuroNext) supports Phase II clinical trials to gather critical information about investigational treatments prior to larger later-stage trials, as well as studies to discover and validate measures of disease and treatment responses that can serve as biomarkers. Since 2011, NeuroNEXT has supported ten studies of biomarkers or therapies for a range of common and rare neurological disorders.
- Strategies to Innovate EmeRgENcy Care Clinical Trials Network (SIREN), led by NINDS and the National Heart Lung and Blood Institute (NHLBI), conducts clinical trials in emergency care for neurologic, cardiac, respiratory, and hematologic conditions. Ongoing trials are testing treatments for TBI and for improving neurological outcomes after cardiac arrest with coma. To aid NIH in combatting the coronavirus pandemic, SIREN rapidly implemented a trial of outpatient convalescent plasma for preventing COVID-19 progression.
- The NINDS Common Data Elements (CDE) Program works with researchers, industry, nonprofit, other Federal agencies and, professional organizations to develop data standards for neurological disorders, to foster collaboration and data sharing across studies and improve data quality and integrity. The program has developed CDEs for 23 disease areas, including many with pediatric standards, as well as a common set for use across diseases.
- The Office of Global Health and Health Disparities within DCR directs NINDS support for research on health disparities and minority, community, and global health. An ongoing strategic planning process will guide and strengthen new NINDS investments in these areas.
Budget Policy: The FY 2022 President’s Budget request for the Division of Clinical Research is $172.425 million, an increase of $8.190 million, or 5.0 percent, from the FY 2021 Enacted level.
Division of Extramural Activities
The DEA leads NINDS efforts in research training and career development, workforce development initiatives, and enhancing neuroscience rigor and reproducibility research. These include:
- The Office of Training and Workforce Development directs NINDS extramural programs for research training and career development, including fellowships and mentored awards for individuals and grants for programs at academic institutions. Complementing NIH-wide programs, NINDS initiatives meet unique training needs across career stages in neuroscience research and include national programs for child neurologists and neurosurgeons who wish to pursue research and career development awards for advanced trainees launching independent projects. In addition, the NINDS Landis Mentor Award promotes excellent mentorship in neuroscience research by recognizing and providing funds to dedicated, effective mentors.
- The Office of Programs to Enhance Neuroscience Workforce (OPEN) represents NINDS at all levels of NIH in matters pertaining to training and workforce development in the extramural biomedical workforce. OPEN develops and implements individual and institutional funding opportunities while working across the NINDS, Blueprint, and BRAIN scientific portfolios to promote the scientific workforce in the neurosciences. OPEN develops training opportunities and organizes conferences, workshops, symposia, and professional development activities to enhance participation in the neuroscience workforce.
- The Office of Research Quality promotes rigor and transparency in neuroscience research and its efforts have been instrumental to policies set by NIH and research journals to improve rigor in experimental design and require transparent reporting in publications. To address needs for training in rigorous research practices, the office is developing a comprehensive educational platform for use by academic institutions and researchers at all career stages.
Budget Policy: The FY 2022 President’s Budget request for the Division of Extramural Activities is $108.8 million, an increase of $5.2 million, or 5.1 percent, from the FY 2021 Enacted level.
Intramural Research Program (IRP)
The NINDS IRP conducts research and research training on the NIH campus and hosts core facilities providing access to state-of-the-art research technologies. The Program spans basic, translational, and clinical research in neuroscience, neurology, and neurosurgery.
- More than 150 laboratories from NINDS and 10 other Institutes conduct neuroscience research at NIH, creating a rich environment for innovative, multidisciplinary studies. Many of these laboratories are housed together in the Porter Neuroscience Research Center, a space designed to encourage collaboration. NINDS has been a leader in coordinating intramural neuroscience researchers’ return to work during the SARS-CoV-2 pandemic.
- NINDS clinical research benefits from the NIH Clinical Center, a hospital solely devoted to clinical investigation. NINDS leads a multisystem study on post-infectious ME/CFS to identify clinical and biological markers and disease mechanisms. Additional clinical studies focus on neurologic aspects of COVID-19, epilepsy, multiple sclerosis, neurodegenerative diseases, brain tumors, movement disorders, and other areas; and they include early trials for drugs, devices, and gene therapy. Through the NIH Undiagnosed Diseases Program, NINDS helps find the causes of puzzling neurological cases referred to the Clinical Center. NINDS also works with two local emergency departments on studies of acute stroke and TBI.
- The unique resources of the IRP enable innovative studies that bridge basic and clinical neuroscience to answer fundamental questions about the nervous system and its diseases. IRP investigators have recently received prestigious research awards: Richard Youle, Ph.D., received the 2021 Breakthrough Prize in Life Sciences for showing how mutations in the genes PINK1 and Parkin harm the brain; Sonja Scholz, M.D., Ph.D., received the American Neurological Association’s 2020 Soriano Lectureship for her use of advanced genetics to study neurodegenerative disorders; and Michael Ward, M.D., Ph.D., is a new awardee of the Chan Zuckerberg Initiative’s Neurodegeneration Challenge Network. A new IRP program will incentivize more high risk, high reward collaboration across basic and clinical research. NINDS also joined NIA to establish a new Center for Alzheimer’s and Related Dementias.
Budget Policy: The FY 2022 President’s Budget request for the Intramural Research Program is $234.3 million, an increase of $8.6 million, or 3.8 percent, from the FY 2021 Enacted level.
Research Management and Support (RMS)
RMS activities provide administrative, budgetary, logistical, and scientific support in the review, award, and monitoring of research grants, training awards, and research and development contracts. RMS also encompasses strategic planning, coordination, and evaluation of NINDS programs, regulatory compliance, and liaison with other Federal agencies, Congress, and the public, as well as activities to communicate research advances and disseminate information about neurological disorders to the public. For example, in 2021, NINDS will launch an update of its Mind Your Risks public health campaign, based on growing evidence linking high blood pressure and the risk of developing dementia. The campaign aims to reach at-risk audiences with messages on the importance of controlling hypertension to prevent stroke and possibly dementia.
Budget Policy: The FY 2022 President’s Budget request for NINDS Research Management and Support is $105.1 million, an increase of $9.7 million, or 10.2 percent, from the FY 2021 Enacted level.
NIH Helping to End Addiction Long-termSM (HEAL) Initiative
NINDS is a leading partner in the NIH HEAL InitiativeSM, launched in 2018 as an aggressive, trans-agency effort to speed scientific solutions to stem the national opioid crisis. HEAL focuses on improving prevention and treatment for opioid misuse and addiction and enhancing pain management, including through the development of nonaddictive alternatives to opioid pain medications. NINDS leads HEAL programs for the preclinical discovery and development of new medications and devices to treat acute and chronic pain conditions, and for evaluating the effectiveness of therapies in clinical trials. Many of these programs build on NINDS and other NIH programs and infrastructure for basic, translational, and clinical research. They include:
- Discovery and Validation of Novel Targets for Safe and Effective Pain Treatment: to find new targets for pain treatment development
- Preclinical Screening Platform for Pain (PSPP): to provide a platform to profile non-opioid, nonaddictive pain therapeutics in validated models relevant to human pain conditions
- Optimization of Non-addictive Therapies to Treat Pain: to accelerate promising small molecule and biologic candidate therapies towards clinical trials
- Discovery and Validation of Biomarkers, Biomarker Signatures, and Endpoints for Pain Indications: to find biomarkers and endpoints for pain to facilitate the development of non-opioid pain therapeutics from discovery through Phase II clinical trials
- Early Phase Pain Investigation Clinical Network (EPPIC-Net): to provide academic and industry researchers with infrastructure for early phase clinical trials of novel pain treatments
- Translational Devices to Treat Pain: to support preclinical development and demonstration of safety and effectiveness for therapeutic and diagnostic devices
- Pain Management Effectiveness Research Network: to compare the effectiveness of existing nonaddictive pain therapies or existing or new prevention and management approaches
Budget Policy: The FY 2022 President’s Budget request for NINDS HEAL funding is $405.4 million, an increase of $135.1 million, or 50.0 percent, from the FY 2021 Enacted level. NINDS HEAL funding for FY 2022 includes $373.8 million for extramural research, $20.0 million for intramural research, and $11.6 million for RMS.
Appropriations History
| Fiscal Year | Budget Estimate to Congress | House Allowance | Senate Allowance | Appropriation |
|---|---|---|---|---|
| 2013 | $1,624,707,000 | $1,629,631,000 | $1,626,365,349 | |
| Rescission | $3,252,731 | |||
| Sequestration | ($81,632,357) | |||
| 2014 | $1,642,619,000 | $1,631,703,000 | $1,587,982,000 | |
| Rescission | $0 | |||
| 2015 | $1,608,461,000 | $1,605,205,000 | ||
| Rescission | $0 | |||
| 2016 | $1,660,375,000 | $1,656,334,000 | $1,694,758,000 | $1,696,139,000 |
| Rescission | $0 | |||
| 20171 | $1,695,180,000 | $1,751,049,000 | $1,803,306,000 | $1,783,654,000 |
| Rescission | $0 | |||
| 20182 | $1,355,998,000 | $1,853,011,000 | $1,904,666,000 | $2,188,149,000 |
| Rescission | $0 | |||
| 20192 | $1,838,556,000 | $2,228,780,000 | $2,275,580,000 | $2,274,413,000 |
| Rescission | $0 | |||
| 20202 | $2,026,031,000 | $2,385,571,000 | $2,490,494,000 | $2,444,687,000 |
| Recission | $0 | |||
| 20212 | $2,245,110,000 | |||
| Recission | ||||
| 20222 | $2,783,300,000 |
1 Budget Estimates to Congress includes mandatory financing.
2 Includes funds derived by transfer from the NIH Innovation Account under the 21st Century Cures Act.
Authorizing Legislation
| PHS Act/ Other Citation | U.S. Code Citation | 2021 Amount Authorized | FY 2021 Enacted | 2022 Amount Authorized | F Y 2022 President's Budget | |
|---|---|---|---|---|---|---|
| Research and Investigation | Section 301 | 42§241 | Indefinite | $2,510,913,000 | Indefinite | $2,783,300,000 |
| National Institute of Neurological Disorders and Stroke | Section 401(a) | 42§281 | Indefinite | Indefinite | ||
| Total, Budget Authority | $2,510,913,000 | $2,783,300,000 |
Amounts Available for Obligation 1
(Dollars in Thousands)
| Source of Funding | FY 2020 Final | FY 2021 Enacted | FY 2022 President's Budget |
|---|---|---|---|
| Appropriation2 | $2,444,687 | $2,513,393 | $2,783,300 |
| OAR HIV/AIDS Transfer | 1,890 | -2,480 | 0 |
Subtotal, adjusted budget authority
| $2,446,577
| $2,510,913
| $2,783,300
|
Subtotal, adjusted budget authority
| $2,442,216
| $2,517,496
| $2,783,300
|
| Total obligations | $2,442,219 | $2,517,496 | $2,783,300 |
1 Excludes the following amounts (in thousands) for reimbursable activities carried out by this account:
FY 2020 - $21,040 FY 2021 - $23,000 FY 2022 - $23,665
2 Of which $70.0 million in FY 2019, $70.0 million in FY 2020, and $50.0 million in FY 2021, and $76.0 million in FY 2022 is derived by transfer from the NIH Innovation Account under the 21st Century Cures Act.
3 Reflects 21st Century Cures Act funding not obligated in FY 2020, and carried over into FY 2021.
Budget Authority by Object Class 1
(Dollars in Thousands)
| FY 2021 Enacted | FY 2022 President's Budget | FY 2022 +/- FY 2021 Enacted | |
|---|---|---|---|
| Total compensable workyears: | |||
| Full-time equivalent | 549 | 607 | 58 |
| Full-time equivalent of overtime and holiday hours | 0 | 0 | 0 |
| Average ES salary | $199 | $205 | $5 |
| Average GM/GS grade | 12.6 | 12.6 | 0.0 |
| Average GM/GS salary | $131 | $134 | $4 |
| Average salary, grade established by act of July 1, 1944 (42 U.S.C. 207) | $111 | $114 | $3 |
| Average salary of ungraded positions (in whole dollars) | $150 | $154 | $4 |
OBJECT CLASSES | FY 2021 Enacted | FY 2022 President's Budget | FY 2022+/- FY 2021 |
|---|---|---|---|
| Personnel Compensation: | |||
| 11.1 Full-time permanent | $39,918 | $45,747 | $6,828 |
| 11.3 Other than full-time permanent | 26,164 | 26,759 | 595 |
| 11.5 Other personnel compensation | 2,180 | 2,229 | 50 |
| 11.7 Military personnel | 431 | 443 | 12 |
| 11.8 Special Personnel Services Payments | 8,995 | 9,200 | 205 |
| 11.9 Subtotal Personnel Compensation | $76,688 | $84,378 | $7,690 |
| 12.1 Civilian Personnel benefits | 25,003 | 28,114 | 3,111 |
| 12.2 Military Personnel Benefits | 356 | 366 | 10 |
| 13.0 Benefits to Former Personnel | 0 | 0 | 0 |
| Subtotal, Pay Costs | $102,048 | $112,858 | $10,810 |
| 21.0 Travel and Transportation of Persons | 580 | 1,191 | 610 |
| 22.0 Transportation of Things | 289 | 294 | 5 |
| 23.1 Rental Payments to GSA | 1 | 1 | 0 |
| 23.2 Rental Payments to Others | 52 | 53 | 1 |
| 23.3 Communications, Utilities and Miscellaneous Charges | 640 | 651 | 12 |
| 24.0 Printing & Reproduction | 3 | 3 | 0 |
| 25.1 Consulting Services | 60,075 | 62,757 | 2,682 |
| 25.2 Other Services | 47,948 | 48,452 | 504 |
| 25.3 Purchase of goods and services from government accounts | 173,376 | 181,755 | 8,379 |
| 25.4 Operation & Maintenance of Facilities | 2,454 | 2,488 | 34 |
| 25.5 R&D Contracts | 34,795 | 42,594 | 7,798 |
| 25.6 Medical Care | 252 | 262 | 9 |
| 25.7 Operation & Maintenance of Equipment | 4,184 | 4,257 | 73 |
| 25.8 Subsistence & Support of Persons | 0 | 0 | 0 |
| 25.0 Subtotal Other Contractual Services | $323,084 | $342,564 | $19,480 |
| 26.0 Supplies & Materials | $14,672 | $14,934 | $262 |
| 31.0 Equipment | 9,897 | 10,075 | 178 |
| 32.0 Land and Structures | 4,438 | 4,518 | 80 |
| 33.0 Investments & Loans | 0 | 0 | 0 |
| 41.0 Grants, Subsidies & Contributions | 2,055,209 | 2,296,158 | 240,949 |
| 42.0 Insurance Claims & Indemnities | 0 | 0 | 0 |
| 43.0 Interest & Dividends | 1 | 1 | 0 |
| 44.0 Refunds | 0 | 0 | 0 |
| Subtotal Non-Pay Costs | $2,408,865 | $2,670,442 | $261,577 |
| Total Budget Authority by Object Class | $2,510,913 | $2,783,300 | $272,387 |
1Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
Salaries and Expenses
(Dollars in Thousands)
| OBJECT CLASSES | FY 2021 Enacted | FY 2022 President's Budget | FY 2022 +/- FY 2021 |
|---|---|---|---|
| Personnel Compensation: | |||
| Other Contractual Services: | |||
| Full-Time Permanent (11.1) | $38,918 | $45,747 | $6,828 |
| Other Than Full-Time Permanent (11.3) | 26,164 | 26,759 | 595 |
| Other Personnel Compensation (11.5) | 2,180 | 2,229 | 50 |
| Military Personnel (11.7) | 431 | 443 | 12 |
| Special Personnel Services Payments (11.8) | 8,995 | 9,200 | 205 |
| Subtotal Personnel Compensation (11.9) | $76,688 | $84,378 | $7,690 |
| Civilian Personnel Benefits (12.1) | $25,003 | $28,114 | $3,111 |
| Military Personnel Benefits (12.2) | 356 | 366 | 10 |
| Benefits to Former Personnel (13.0) | 0 | 0 | 0 |
| Subtotal Pay Costs | $102,048 | $112,858 | $10,810 |
| Travel & Transportation of Persons (21.0) | $580 | $1,191 | $610 |
| Transportation of Things (22.0) | 289 | 294 | 5 |
| Rental Payments to Others (23.2) | 52 | 53 | 1 |
| Communications, Utilities and Misc. Charges (23.3) | 640 | 651 | 12 |
| Printing and Reproduction (24.0) | 3 | 3 | 0 |
| Consultant Services (25.1) | 59,322 | 61,990 | 2,669 |
| Other Services (25.2) | 47,948 | 48,452 | 504 |
| Purchases from government accounts (25.3) | 107,614 | 111,962 | 4,348 |
| Operation and Maintenance of Facilities (25.4) | 2,454 | 2,488 | 34 |
| Operation and Maintenance of Equipment (25.7) | 4,184 | 4,257 | 73 |
| Subsistence and Support of Persons (25.8) | 0 | 0 | 0 |
| Subtotal Other Contractual Services | $221,522 | $229,149 | $7,627 |
| Supplies and Materials (26.0) | $14,672 | $14,934 | $262 |
| Subtotal Non-Pay Costs | $237,758 | $246,275 | $8,517 |
| Total Administrative Costs | $339,805 | $359,133 | $19,327 |
Detail of Full-Time Equivalent Employment (FTE's)
| OFFICE/DIVISION | FY 2020 Final | FY 2021 Enacted | FY 2022 President's Budget | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Civilian | Military | Total | Civilian | Military | Total | Civilian | Military | Total | |
| Office of the Director | |||||||||
| Division of Clinical Research | |||||||||
| Division of Translational Research | |||||||||
| Division of Intramural Research | |||||||||
| Division of Extramural Activities | |||||||||
| Division of Neuroscience | |||||||||
| Includes FTEs whose payroll obligations are supported by the NIH Common Fund. | |||||||||
| Direct: | 74 | - | 74 | 75 | - | 75 | 83 | - | 83 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 74 | - | 74 | 75 | 75 | 83 | - | 83 | |
| Direct: | 17 | - | 17 | 19 | - | 19 | 20 | - | 20 |
| Reimbursable: | 1 | - | 1 | 1 | - | 1 | 1 | - | 1 |
| Total: | 18 | - | 18 | 20 | - | 20 | 21 | - | 21 |
| Direct: | 24 | - | 24 | 27 | - | 27 | 31 | - | 31 |
| Reimbursable: | 3 | - | 3 | 3 | - | 3 | 3 | - | 3 |
| Total: | 27 | - | 27 | 30 | - | 30 | 34 | - | 34 |
| Direct: | 282 | 4 | 286 | 296 | 4 | 300 | 332 | 5 | 337 |
| Reimbursable: | 14 | - | 14 | 14 | - | 14 | 14 | - | 14 |
| Total: | 296 | 4 | 300 | 310 | 4 | 314 | 346 | 5 | 351 |
| Direct: | 58 | - | 58 | 60 | - | 60 | 61 | - | 61 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 58 | - | 58 | 60 | - | 60 | 61 | - | 61 |
| Direct: | 44 | - | 44 | 46 | - | 46 | 53 | - | 53 |
| Reimbursable: | 4 | - | 4 | 4 | - | 4 | 4 | - | 4 |
| Total: | 48 | - | 48 | 50 | - | 50 | 57 | - | 57 |
| Total | 521 | 4 | 525 | 545 | 4 | 549 | 602 | 5 | 607 |
| FTEs supported by funds from Cooperative Research and Development Agreements | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
FTEs supported by funds from Cooperative Research and Development Agreements.
| FISCAL YEAR | Average GS Grade |
|---|---|
| 2018 | 12.5 |
| 2019 | 12.6 |
| 2020 | 12.6 |
| 2021 | 12.6 |
| 2022 | 12.6 |
Detail of Positions1
(Dollars in Thousands)
| GRADE | FY 2020 Final | FY 2021 Enacted | FY 2022 President's Budget |
|---|---|---|---|
| Total, ES Positions | 1 | 1 | 1 |
| Total, ES Salary | 197,300 | 199,273 | 204,653 |
| GM/GS-15 | 57 | 66 | 68 |
| GM/GS-14 | 78 | 82 | 96 |
| GM/GS-13 | 98 | 102 | 113 |
| GS-12 | 60 | 62 | 63 |
| GS-11 | 19 | 21 | 22 |
| GS-10 | 2 | 2 | 2 |
| GS-9 | 14 | 15 | 15 |
| GS-8 | 5 | 6 | 6 |
| GS-7 | 6 | 5 | 5 |
| GS-6 | 1 | 1 | 1 |
| GS-5 | 3 | 3 | 3 |
| GS-4 | 3 | 3 | 3 |
| GS-3 | 1 | 1 | 1 |
| GS-2 | 3 | 3 | 3 |
| GS-1 | 0 | 1 | 1 |
| Subtotal | 350 | 373 | 402 |
| Grades established by Act of July 1, 1944 (42 U.S.C. 207): | |||
| Assistant Surgeon General | 0 | 0 | 0 |
| Director Grade | 0 | 0 | 0 |
| Senior Grade | 0 | 0 | 0 |
| Full Grade | 4 | 4 | 5 |
| Senior Assistant Grade | 0 | 0 | 0 |
| Assistant Grade | 0 | 0 | 0 |
| Subtotal | 4 | 4 | 5 |
| Ungraded | 206 | 209 | 209 |
| Total permanent positions | 358 | 364 | 398 |
| Total positions, end of year | 561 | 587 | 617 |
| Total full-time equivalent (F T E) employment, end of year | 525 | 549 | 607 |
| Average ES salary | 197,300 | 199,273 | 204,653 |
| Average GM/GS grade | 12.6 | 12.6 | 12.6 |
| Average GM/GS salary | 129,249 | 130,541 | 134,066 |
1 Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
