
Michelle Gray, Ph.D.
Assistant Professor
Department of Neurology
Center for Neurodegeneration and Experimental Therapeutics
University of Alabama at Birmingham
http://labs.uab.edu/mccgray/
Leveraging your strengths into a robust research program
I was raised in a small rural community in West Central Alabama. It was there that I developed a love of all the animal life surrounding me, and it inspired me to major in biology as an undergraduate at Alabama State University. My entire career—from undergrad to now—I have been supported by NIH in some fashion. Funding from NIH has really been instrumental in my success throughout my career.
As an undergraduate at Alabama State University, I knew I liked science but I didn’t know if my path would be medicine or research. I participated in the NIH-funded programs Minority Biomedical Research Support (now split into the RISE, SCORE, and IMSD programs) and Minority (now “Maximizing”) Access to Research Careers (MARC). These two programs got me working in the lab and even paid for me to spend a summer at University of Wisconsin Madison doing research. Through these experiences, I realized that my questioning nature would be a good fit for research. The programs also gave me confidence in my scientific abilities.
I then went on to an interdisciplinary Molecular, Cellular and Developmental Biology doctoral program at The Ohio State University. I joined the lab of Dr. Christine Beattie as her first graduate student. I received an NINDS F31 Ruth L. Kirschstein National Research Service Award to support my work on nervous system development using the zebrafish as a model organism. This award was extremely helpful for my training, as my primary advisor was brand new and had no other source of support for my project besides her startup funds. The F31 allowed me to focus on completing an independent research project without having to seek other avenues of support. The award also funded my travel to conferences, such as the Society for Neuroscience Annual Meeting, that my lab didn’t regularly attend but were relevant to my career.
I decided to change the focus of my work for my postdoctoral training and study neurodegenerative diseases. I believed that what I had learned as a developmental neurobiologist could be used to provide novel insights into diseases that caused neurodegeneration. I joined X. William Yang’s lab at the University of California, Los Angeles to learn the technical skills and rationale required for generating mouse models of neurodegenerative diseases. We developed a conditional Bacterial Artificial Chromosome transgenic mouse (BACHD) that contains the entire human huntingtin genomic locus modified to include the Huntington’s disease (HD) causing mutation. These mice have proven to be very useful in determining cell-type specific contributions to HD pathogenesis and are now widely used throughout the world. While in the Yang lab, I was supported on T32 Institutional Training Grants from NINDS on Neurobehavioral Genetics and from NICHD on Intellectual and Developmental Disabilities.
As my postdoctoral training was wrapping up, I began thinking about what my research question was going to be, and how I would distinguish myself from my postdoc advisor as an independent scientist. At that time, the HD field was very neuron-centric, with a strong focus on medium spiny neurons in the basal ganglia. The role of astrocytes in HD was a novel area with very little research. I knew that my technical training as a molecular neurobiologist had prepared me well to fill this niche—I just needed to learn about glial biology.
At the University of Alabama at Birmingham (UAB) I found the perfect fit: the Center for Glial Biology in Medicine, which contained one of the largest groups of glial biologists in the US, and the Department of Neurology’s Center for Neurodegeneration and Experimental Therapeutics (CNET), to support my further understanding of glial cell contribution to neurodegeneration.
I started in an Instructor position with the goal of studying astrocytes and their contribution to Huntington’s Disease. Dr. David Standaert, who was the director of CNET (now chair of the department) and a clinician-scientist with expertise in movement disorders and neurodegenerative diseases encouraged me to apply for the NINDS K01 Career Development Award. The award was well-suited for my situation because I had a strong scientific background, an interesting research question, and was looking to get additional training in a new area—glial biology. Joining Dr. Standaert as my co-mentor was an expert in astrocyte biology, Dr. Vladimir Parpura. Together, they helped my science grow and taught me how to manage my new responsibilities including guiding students, staffing my laboratory, and expanding my research.
After obtaining this award, I was promoted to a tenure-track Assistant Professor position. The K01 has helped me gain experience in a new field, to take classes, to be mentored by senior scientists, to participate in local and national conferences, and provided me with protected time for building my research program. The data that I gathered during the K01 served as preliminary data for my first R01, awarded in 2015. This preliminary data involved time-consuming studies (whose outcome was not guaranteed) to generate double transgenic mice—studies I might not have been able to do without the K funding. If I had been forced to focus on applying for funding and keeping my lab going, I wouldn’t have had time to learn the new skills that have been critical to my career development and progression. I am very grateful to the NIH for this opportunity and encourage others to take advantage of it.
Current Research
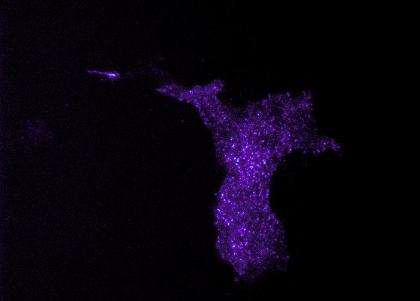
My primary research focus is clarifying the contribution of astrocytes, a type of brain cell, to the development of Huntington’s disease (HD). I use a mouse model of HD that I developed as a postdoctoral fellow to investigate whether decreasing expression of the mutant huntingtin gene in astrocytes can restore function to these mice. We have identified an improvement in motor and psychiatric-like symptoms and improvement in neuropathological signs. We are continuing our studies of astrocyte involvement in HD by exploring the effect of modulation of gliotransmitter release from astrocytes on HD signs and symptoms. The image shows TIRF imaging of primary cortical astrocyte transfected with fluorescently tagged vGLUT3 to visualize vesicle movement.
