The goal of the R35 (R35; RPA; RFA-NS-22-038) is to help investigators make meaningful contributions to neuroscience by providing greater funding stability, flexibility, and support for your overall research project. Use this page to learn more about the R35 program and associated policies and procedures.
What is the R35 Research Program Award (RPA)?
The R35 RPA is a funding mechanism that supports your research efforts by:
Learn More about Eligibility for the R35 RPA
- View the short video on eligibility
- Contact your Program Director for more information.
R35 Policies for Awardees
Policy for Requesting Budget Increase After Year 5
- See Notice NOT-NS-24-124 for the procedure
R35 No Cost Extension Policy
- Contact the NINDS Grants Management Specialist and program officer indicated in eRA commons for this award
R35 Frequently Asked Questions
Q. Who is eligible to apply?
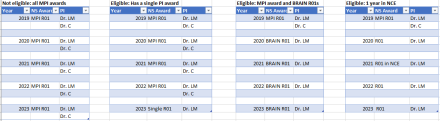
A. Eligibility to apply to this NOFO is limited to PDs/PIs who meet all the following criteria:
a. PDs/PIs who have had at least one of the following types of active NINDS grants in each of the past 5 years, that is FY20-24: R00, R01, R35, R37, R56, DP1, DP2. If you have more than one year in a no cost extension, briefly justify.
PDs/PIs whose 5-year NINDS funding history is limited to a only MPI or multi-component grants, and do not have a single PI NINDS grant, are not eligible to apply.
b. At some point in the previous five years, my grant was either in an NCE or I had an NINDS R56 award. Do I still qualify has having had continuous NINDS support for the last five years? Yes. Continuous support from NINDS on an active R01 equivalent grant (including those on an NCE or R56) qualifies as five years of consecutive support.
Q. Could you provide an overview and template for calculating my budget?
A. Applicants may request up to a maximum of $750,000 direct costs per year; however, the requested RPA budget must be commensurate with the investigator's recent level of NINDS support. That level of support must be calculated using the average direct cost total for the 4 most recent years (i.e. 2020 - 2023 or 2021 - 2024, whichever is greater) from all awarded NINDS research grants of the type that will be folded into the R35 award; it should not include funding that is exempt from R35 fold in (see Section 1, Overview). Calculations should use awarded direct costs (including administrative cuts), and in the case of MPD/MPI awards or multi-component grants/cooperative agreements, should only include the amount of the total award that is attributable to the applicant PD/PI. Moreover, in the case of multi-component grants/cooperative agreements, the budget should not incorporate funds attributable to cores, as these funds will not be folded into the RPA. Please note that pending support should not be considered when developing an RPA budget, even if such applications should get funded between the R35 submission and award. Although the RPA mechanism is not intended to support the expansion of an investigator's current level of NINDS funding, RPAs will be awarded for no less than $350K DC. Barring this exception, only in rare and extremely well-justified circumstances (e.g. an investigator is still within the growth phase of their careers) will awards exceed the most recent 4-year funded average of NINDS grants subject to be folded in. Applications submitted with budgets of more than 20% over the 4 year average described above will be considered non-compliant to this NOFO and will be withdrawn prior to review. PDs/PIs are encouraged to discuss their proposed budgets / projects with NINDS program staff prior to submission and should provide detailed evidence supporting their proposed request in the budget justification section (e.g. table calculating 4-year average of direct costs, including grant numbers with awarded direct costs for each year; for example, see NINDS Research Program Award (R35). Year one of the R35 award will be offset by any funds disbursed in the same FY from awards that will be folded into the RPA.
| Project Num | FY | Awd Dir Cost | DC/PI |
|---|---|---|---|
| R01NSxxxxxx-xx | 2020 | $218,750 | $218,750 |
| R01NSxxxxxx-xx | 2021 | $225,878 | $225,878 |
| R01NSxxxxxx-xx | 2022 | $280,328 | $280,328 |
| R01NSxxxxxx-xx | 2023 | $153,553 | $153,553 |
| R01NSxxxxxx-xx | 2023 | $280,328 | $280,328 |
| R21NSxxxxxx-xx | 2023 | $275,000 | $68,750 |
| R21NSxxxxxx-xx | 2023 | $225,000 | $112,500 |
| R01NSxxxxx-xx | 2024 | $276,203 | $276,203 |
| R21NSxxxxxx-xx | 2024 | $275,000 | $68,750 |
| R21NSxxxxxx-xx | 2024 | $225,000 | $112,500 |
| 2020 | 2021 | 2022 | 2023 | 2024 | Average FY21-24 | 120% |
|---|---|---|---|---|---|---|
| $218,750 | $225,878 | $280,328 | $615,131 | $457,453 | $394,698 | $473,637 |
I. Funding Opportunity Description
Q. What is the NINDS Research Program Award and what is its purpose?
The Research Program Award (RPA) is a funding mechanism being used by the NINDS to provide support for a single investigator and his/her laboratory as a whole, rather than on a project-by-project basis. Its purpose is to support all NINDS mission-relevant research in a laboratory through a single grant award that will be up to eight years in duration.
Q. What distinguishes the RPA from traditional NIH programs?
The RPA is intended to provide an investigator with funding stability and increased flexibility to pursue new research directions, and enable longer-range research goals. Other differences include: a reduced level of detail required in the application, including the elimination of specific aims; differences in the review process and review criteria to emphasize the impact of the work and to de-emphasize details of the approach.
Q. How can I decide if RPA is right for my circumstances?
Assess how much time and effort it takes you to maintain the current NINDS funding of your laboratory through multiple, separate grant applications. Decide whether consolidation of your projects at something close to your average level of NINDS support is worth the advantages provided by the RPA program, which includes up to eight years of stable funding. Consult with your current Program Director for additional guidance.
Q. How much time/effort must recipients devote to RPA? How many calendar months?
By replacing all or most of an investigator’s NINDS funding, the research supported by an RPA should be the major focus of the PD/PI’s laboratory or research group. Therefore, the PD/PI must devote at least 6 person months (i.e., the equivalent of 50% effort on for a full-year appointment, 66.67% on a 9-month appointment, or 100% on a 6-month appointment) to the RPA throughout the duration of the award period. PDs/PIs will be expected to renegotiate their time and effort on other non-NINDS awards if necessary.
Q. What scientific areas of research are appropriate for support by RPA?
Any research area within the mission of NINDS is eligible for support using the RPA award. However, some types of research might be more suitably supported through a traditional R01 or other grant mechanism that requires more specific methodological detail or programmatic oversight (e.g., clinical trials, advanced translational research). NINDS strongly encourages applicants to speak with NINDS program staff to help determine if a different mechanism is better suited to the proposed research area. The RPA is neither appropriate for the support of clinical trials designed to answer specific questions about the safety, tolerability, efficacy, effectiveness, clinical management, and/or implementation of pharmacologic, behavioral, biologic, surgical, or device (invasive or non-invasive) interventions nor is it appropriate for research that has been traditionally supported by another NIH Institute or Center, even when combined as part of a larger research program.
Q. Can technology development be included or is the award only for hypothesis-driven research?
Yes. Technology development can be an essential part of any research program and hypothesis-driven science is only one way of approaching a research problem. However, applicants should contact NINDS staff to determine if the R35 is the most appropriate mechanism of support, as NINDS has a number of Funding Opportunities that specifically support tool and technology development and dissemination.
Q. Can you clarify how much flexibility is meant by "flexibility to pursue new research directions"?
With an RPA, PD/PIs will be allowed to pursue new research objectives and problems and to modify their model systems or approaches, but will be expected to work with program staff to ensure that the program remains within the mission of the NINDS and compliant with NIH policy.
Q. What will be considered in scope and what would be out of the scope of an RPA?
Work that migrates fully into the mission of another NIH institute or center would be considered out of scope and not appropriate for pursuit through the RPA. Changes in scope could include, for example, the addition of or a change in the approved use of human subjects, vertebrate animals, select agents or human embryonic stem cells (hESCs). Such changes require prior approval by NINDS before the work is initiated. However, such changes in scope can be approved with appropriate documentation. Please refer to the NIH Grants Policy Statement for additional guidance (NIH GPS 8.1.2.5).
Q. How many RPAs does NINDS expect to award in FY 2025?
This depends on the number and cost of meritorious applications. NINDS estimates that as many as 25 awards will be made.
II. Eligibility Information
Q. Who is eligible to apply?
Eligibility to apply to this NOFO is limited to PDs/PIs who meet all the following criteria:
a. PDs/PIs who have had at least one of the following types of active NINDS grants in each of the past 5 years (that is FY20-24), with no more than one of those years in a no cost extension: R00, R01, R35, R37, R56, DP1, DP2.
PDs/PIs whose 5-year NINDS funding history is limited to a only MPI or multi-component grants, and do not have a single PI NINDS grant, are not eligible to apply.
b. At some point in the previous five years, my grant was either in an NCE or I had an NINDS R56 award. Do I still qualify has having had continuous NINDS support for the last five years? Yes. Continuous support from NINDS on an active R01 equivalent grant (including those on an NCE or R56) qualifies as five years of consecutive support.
Q. What is the definition of a PD/PI on a NINDS grant in the context of “received continuous R01 or R01 equivalent grant support from NINDS for at least the past 5 years”?
You must be listed as the PD/PI on the Notice of Award (NoA) of an NINDS grant to be considered the PD/PI. You must have been funded continuously as PD/PI in each of the past five consecutive years at the time of application submission.
Q. Is there a separate NOFO for so-called “emerging” or early career investigators?
No. Investigators at all career stages that meet the eligibility requirements are encouraged to apply for the RPA. In developing a pay plan for the RPA, NINDS will seek to ensure that award recipients reflect varying career stages.
Q. May two or more scientists apply as a team for an NINDS RPA?
No. This NOFO is intended to provide support for the research program of a single independent, investigator.
Q. Are scientists in the NIH intramural research program eligible to apply?
No. NIH intramural research program scientists are not eligible to apply. An RPA application can include collaboration with an intramural scientist, but no funds can be provided to the intramural laboratory via the RPA.
Q. Are individuals who have support from sources other than NINDS eligible to apply?
Yes. Individuals may have support from other NIH components or from other sources, provided they meet all other eligibility requirements and are able to commit at 6 person months (i.e., the equivalent of 50% effort on for a full-year appointment, 66.67% on a 9-month appointment, or 100% on a 6-month appointment) to the RPA throughout the duration of the award period.
Q. Are Howard Hughes Medical Institute investigators eligible to apply for RPA? What about individuals with other types of substantial, unrestricted research support?
Yes. HHMI investigators are eligible to apply for RPA, provided they are able to commit 6 person months (i.e., the equivalent of 50% effort on for a full-year appointment, 66.67% on a 9-month appointment, or 100% on a 6-month appointment) to the RPA throughout the duration of the award period. The relationship between the work supported by another organization and the work to be supported by NINDS must be distinct, and it must be clearly explained how the funding provided by the RPA will impact your research program. Investigators with other types of substantial, unrestricted laboratory support will be similarly considered on a case-by-case basis, however, lower funding priority may be given to applicants with significant unrestricted research support.
Q. If I have an appointment at more than one institution, can I apply for a separate RPA through each institution?
No. An investigator can only receive one RPA award, which should be submitted by the institution where he or she primarily conducts his or her research program.
Q. Can I get a definitive determination of my eligibility prior to submitting an application to this Request for Application (RFA)?
No. NINDS staff can give you advice on eligibility; however, applications are evaluated for eligibility by the Receipt and Referral Office at the NIH Center for Scientific Review based on the criteria stated in the NOFO.
Q. Can I submit an R01 application and apply for RPA at the same time?
Yes. You may submit an R01 application in parallel to your R35 application; however, if both applications receive meritorious scores only one of these applications will be supported.
III. Application and Submission information
Q. Is there a limit on the number of applications that can be submitted by an institution? By a PD/PI?
There is no limit on the number of applications that can be submitted by an institution. A PD/PI may submit only one application for an RPA.
Q. Can I get advice on my ideas for submission of a RPA application?
You may discuss your ideas with the program contact named in the NOFO or with the program director(s) who administers your current NINDS applications and awards.
Q. What format should the application follow?
Follow the instructions in the SF424 R&R application guide as modified by the instructions in the NOFO. Please note that the RPA application has additional requirements in the following sections:
- PHS 398 Research Plan [Research Strategy]
- R&R Budget
- SF424(R&R) Senior/Key Person Profile
- SF424(R&R) Other Project Information
Q. How is funding attributed for Multi-PI awards or Multi-Component awards when calculating the investigator's recent level of NINDS support.
Only the portion of a Multi-PI (MPI) or Multi-Component award that is attributable to the PI will be used. This will be determined by equally dividing dollars among all the research PIs on a project, or using the subaward allocation from the grant if the PIs are at different institutions. For example, if there are two PIs on an MPI award, the award total cost dollars will be divided in half. If the PIs are different institutions with delineated subaward allocation, the subaward amount will be used. If you believe this attribution of funds is incorrect and may erroneously cause you to be subject to the policy, you should contact your Program Director to clarify the situation. At this time, you may be asked to supply Just In Time information documenting your effort on the award(s) in question. Support received by a PI to lead a core facility on a multi-component grant or cooperative agreement will not be considered in these calculations.
RESEARCH PLAN
Q. Why is the Research Strategy Section only six pages?
Because the goal of RPA is to focus investigator and reviewer attention on the higher level questions about significance and impact of the research program, details in the research plan can be reduced.
Q. What became of the specific aims section of the grant application?
Specific aims are not required in order to focus the application on the impact of an investigator’s entire NINDS supported research program.
Q. Should I submit letters of support?
The application must include a letter from the institution’s Authorized Organizational Official (AOR) indicating that they are aware of and accept the condition that other NINDS research awards must be relinquished as a condition of receiving a RPA and providing a statement that if chosen to receive an award, the PD/PI will commit a minimum of 6 person months (as described above) throughout the duration of the RPA. Letters of support should be included for all collaborators.
Q. What is the maximum allowable budget?
Applicants may request up to a maximum of $750,000 direct costs per year; however, the requested RPA budget must be commensurate with the investigator’s recent level of NINDS support.
Q. How should I determine my RPA budget?
Applicants may request up to a maximum of $750,000 direct costs per year; however, the requested RPA budget must be commensurate with the investigator's recent level of NINDS support. That level of support must be calculated using the average direct cost total for the 4 most recent years (i.e. 2020 - 2023 or 2021 - 2024, whichever is greater) from all awarded NINDS research grants of the type that will be folded into the R35 award; it should not include funding that is exempt from R35 fold in (see Section 1, Overview). Calculations should use awarded direct costs (including administrative cuts), and in the case of MPD/MPI awards or multi-component grants/cooperative agreements, should only include the amount of the total award that is attributable to the applicant PD/PI. Moreover, in the case of multi-component grants/cooperative agreements, the budget should not incorporate funds attributable to cores, as these funds will not be folded into the RPA. Please note that pending support should not be considered when developing an RPA budget, even if such applications should get funded between the R35 submission and award. Although the RPA mechanism is not intended to support the expansion of an investigator's current level of NINDS funding, RPAs will be awarded for no less than $350K DC. Barring this exception, only in rare and extremely well-justified circumstances (e.g. an investigator is still within the growth phase of their careers) will awards exceed the most recent 4-year funded average of NINDS grants subject to be folded in. Applications submitted with budgets of more than 20% over the 4 year average described above will be considered non-compliant to this NOFO and will be withdrawn prior to review. PDs/PIs are encouraged to discuss their proposed budgets / projects with NINDS program staff prior to submission and should provide detailed evidence supporting their proposed request in the budget justification section (e.g. table calculating 4-year average of direct costs, including grant numbers with awarded direct costs for each year; for example, see NINDS Research Program Award (R35)). Year one of the R35 award will be offset by any funds disbursed in the same FY from awards that will be folded into the RPA.
Q. Could you provide a template for calculating my budget?
| Project Num | FY | Awd Dir Cost | DC/PI |
|---|---|---|---|
| R01NSxxxxxx-xx | 2020 | $218,750 | $218,750 |
| R01NSxxxxxx-xx | 2021 | $225,878 | $225,878 |
| R01NSxxxxxx-xx | 2022 | $280,328 | $280,328 |
| R01NSxxxxxx-xx | 2023 | $153,553 | $153,553 |
| R01NSxxxxxx-xx | 2023 | $280,328 | $280,328 |
| R21NSxxxxxx-xx | 2023 | $275,000 | $68,750 |
| R21NSxxxxxx-xx | 2023 | $225,000 | $112,500 |
| R01NSxxxxx-xx | 2024 | $276,203 | $276,203 |
| R21NSxxxxxx-xx | 2024 | $275,000 | $68,750 |
| R21NSxxxxxx-xx | 2024 | $225,000 | $112,500 |
| 2020 | 2021 | 2022 | 2023 | 2024 | Average FY21-24 | 120% |
|---|---|---|---|---|---|---|
| $218,750 | $225,878 | $280,328 | $615,131 | $457,453 | $394,698 | $473,637 |
Q. I am supported by a single NINDS modular R01 and would like to apply for and RPA; however I don’t want my application to be withdrawn for being over the 20% threshold. How should I proceed?
No RPA will be awarded for less than $350K DC. Applicants traditionally supported by 1 modular R01 should submit an R35 budget for this amount (with appropriate justification for the increase in budget).
Q. Will salary or other funds I receive as a co-investigator on another PD/PIs NINDS research grant be counted toward the RPA budget? May I continue to receive this support if I am awarded an RPA?
Support as a co-investigator on another PI/PD’s grant will not be counted nor folded into the RPA budget. Such support may continue through the end of the existing grant period. It is expected that the total amount of future sub-contracts will not significantly exceed the amount of support that is currently held by the applicant. Sub contracts should not be used to circumvent the budget limits of the RPA.
Q. I have an application that received a score within the NINDS payline that was reviewed by the NINDS Council at the September 2024 meeting. How will this To Be Paid grant impact my R35 budget if it receives a fundable score?
No pending support (e.g. grants that have not been awarded at the time of your R35 application submission) can be used to determine your RPA budget. At such time that you are offered an R35 award, you should weigh the benefits of 8 years of stable funding against the potential peaks and valleys associated with shorter term awards.
Q. What grants should I include when determining an appropriate budget for my research program?
All grants subject to consolidation into the R35 should be used to calculate your budget. This includes all NINDS supported RPGs, P01s, P50, and other Center awards. There are a number of NINDS activities that are excluded from consolidation and that should not factor into the determination of your R35 budget. These include:
- Contracts;
- SBIR/STTR grants or cooperative agreements;
- Conference grants;
- Grants or cooperative agreements that exclusively support research resources (e.g. P30, R24 or U24 awards);
- Grants or cooperative agreements supporting training and workforce development;
- Grants or cooperative agreements supporting clinical trials submitted in response to NINDS clinical trials required NOFOs;
- Grants or cooperative agreements submitted in response to the NINDS IGNITE, CREATE, or Translational Neural Devices Programs;
- Grants or cooperative agreements submitted in response to BRAIN Initiative NOFOs
Q. How do I factor in my contribution from P01s, P50s or other multi-component awards when determining my RPA budget?
When determining your RPA budget, you should only include the monies directed towards your research efforts on the MPI grant. In the case of P01s or Center awards, you should not include funds directed to either administrative or scientific cores; these funds will not be rolled into the RPA and will remain associated with the multi-component project.
Q. How do I build in annual inflationary increases into my RPA budget if I am asking for the $750K maximum?
All R35 awards will be made with flat budgets. Although there may be an opportunity for a modest increase to your award amount following the 5 year administrative checkpoint, this is not guaranteed, and should not be expected.
Q. My grant was in a no cost extension for one year between 2020 and 2022 (or 2021 – 2023). How do I factor this year into my four year average when determining my R35 budget?
An NCE is an extension of time, and not money. As no new funds were awarded during your NCE year, you should include 1 year of zero dollars in your calculation. For example, if you had a non-modular R01 with a budget of $585,000 DC that was awarded with a 17.5% administrative cut (i.e. $482,625 DC) in FY20, FY21, and FY22 and that grant was in an NCE for FY23, your 4 year average would be $386,100 DC.
Q. In the absence of specific aims, how do I conceptualize and prepare a non-modular budget?
The requested budget should include information for anticipated costs related to accomplishing the Research Strategy proposed in the application. Given that the NINDS RPA will generally fold in an investigator’s current research projects, the requested budget should reflect the budgetary needs for the current research.
Q. Can I request money for equipment in the budget?
Yes. You may request money for equipment in the first year and in any subsequent years with appropriate justification. Such requests should be accommodated within a budget that is commensurate with the overall NINDS grant support of the laboratory in recent years. This is not intended as a mechanism to acquire "big-ticket" items that may be covered under instrument-specific funding opportunities.
Q. Can I continue to work with my current collaborators, including foreign collaborators?
Yes. NINDS strongly endorses collaborative research. However, as the RPA concept is based on the idea that NINDS will provide support to individual investigators and their laboratories, Multi-PD/PI applications are not appropriate for the RPA. Most collaborators will be expected to work together because of their mutual interest in a problem. This applies to foreign collaborations as well. NINDS will accept applications with a foreign collaboration, but will likely not provide significant funding for foreign consortium arrangements.
Q. Can I include a consortium contract for a collaborator?
Yes, but such requests must be extremely well justified, and in general collaborations should be supported by resources provided by both parties, rather than through a subcontract. In rare circumstances, a letter from the subcontract PD/PI should be included, making it clear why he or she cannot participate in collaborative research with the PD/PI without support from RPA.
SENIOR / KEY PERSON PROFILE
Q. Who, if anyone, in addition to the PD/PI should be listed as "key personnel"?
Key personnel include any other individuals who contribute to the scientific development or execution of a project in a substantive, measurable way, whether or not they receive salaries or compensation under the grant. Typically, these individuals have doctoral or other professional degrees, although individuals at the master's or baccalaureate level may be considered senior/key personnel if their involvement meets this definition. Consultants and those with a postdoctoral role also may be considered senior/key personnel if they meet this definition. Senior/key personnel must devote measurable effort to the project whether or not salaries or compensation are requested. "Zero percent" effort or "as needed" are not acceptable levels of involvement for those designated as senior/key personnel. See NIH Grant & Funding FAQ's on Senior & Key Personnel for additional information on the use of key personnel in your application.
Q. How will support of other senior/key personnel (co-PD/PIs) be considered?
Senior/key personnel who are independent investigators can contribute effort toward RPA. They may receive support from the award, but only if they do not also receive support from their own RPA award.
Q. What may I include in the appendix?
Appendix materials are limited to those allowed by NIH Policy. See NOT-OD-17-098.
Q. Who will be the scientific point of contact for my RPA application?
Dr. Alisa Schaefer (Scientific Officer, Division of Extramural Activities) will serve as the initial point of contact for questions about the NOFO. Should you wish to discuss scientific aspects of your application, you should contact your current NINDS program officer. Once applications are received, they will be referred to the most relevant program official based on internal NINDS referral procedures and guidelines. The name and contact information for the program official will appear at the top of the summary statement.
IV. Review Information
Q. How will responses to this NOFO be reviewed?
Review will be by special emphasis panels organized by the NINDS Scientific Review Branch. The name and contact information for the scientific review official assigned to the application will be posted in the Commons.
Q. What is the timeline for application, review and award?
Applications received by July 2024 will be reviewed in November 2024 for consideration by the National Advisory Neurological Disorders and Stroke Council in January 2025, with the earliest possible awards beginning in April 2025.
Q. Will the reviewers have expertise in the subject area of my application?
Yes. NINDS will ensure that reviewers have the relevant expertise to review the application, bearing in mind that RPA is intended to support a broad program of research and the breadth of research areas that are encompassed by the NINDS mission. Thus, reviewers will be expected to bring a broad perspective rather than detailed expertise.
Q. How will the review process for RPA differ from that for regular R01 research grant applications?
Reviewers will be asked to provide a single overall impact score and not to provide individual criterion scores. This is intended to shift emphasis away from details of the application and the approach, and to emphasize the potential impact of the investigator's research program on the field. Reviewers will be provided with information about the applicant’s funding history and asked to comment on whether or not the budget requested in the RPA reflects the current scope of the investigator’s research support from NINDS.
Q. How do the review criteria differ from those for a regular R01 research grant application?
The review criteria are the same, but the wording has been modified to emphasize the review of the investigator's overall NINDS-relevant research program rather than a specific, narrowly-focused project with highly tailored specific aims. Reviewers should emphasize RPA-specific aspects of significance, investigator qualifications, innovation, approach and environment.
Q. How will the study section arrive at a budget recommendation?
Reviewers will be asked to consider whether the budget and the requested period of support are fully justified and reasonable in relation to the proposed work. It is anticipated that reviewers will consider the past productivity of the investigator's laboratory in areas relevant to the NINDS mission, given the resources at the laboratory's disposal, evidence of efficient use of funds in the past and likelihood of efficient use of funds in the future. Reviewers will be provided with information about the applicant’s funding history and asked to comment on whether or not the budget requested in the RPA reflects the current scope of the investigator’s research support from NINDS. If the proposed budget exceeds the current (2020-2023 or 2021-2024) level of support, reviewers will consider whether there are exceptional circumstances that justify the increased level of support. Applications requesting budgets more than 20% over the 4-year average will be considered non-compliant to this NOFO and will not be reviewed.
Q. Can I appeal the review of my RPA application?
No. This NOFO is a one-time request for applications. Therefore, be sure to inform NINDS of any potential reviewer conflicts in a cover letter included with the application at the time of submission.
Q. If my application for RPA is not funded, will I be able to prepare a resubmission?
No, but you may be eligible to submit a new application to another RPA NOFO in future fiscal years.
Q. How does the NIH Special Council Review policy apply to RPA?
In February 2024, NINDS announced modification to the NIH Special Council Review policy (NOT-NS-24-060). These changes effectively lower the funding threshold that requires special review of a pending application by the National Advisory Neurological Disorders and Stroke (NANDS) Council and sets a more stringent payline for pending applications that cause a PD/PI to exceed this threshold. RPA funding will be factored into the calculation of an investigator’s “research support” should additional NIH funding be sought.
Q. What will be Council involvement in the second level peer review of RPA applications?
RPA applications in response to this NOFO will be made available to the National Advisory Neurological Disorders and Stroke Council in the same way as other applications. Council members will be free to comment on any application and will vote to approve applications for potential funding.
V. Awardee Information
Q. How will NINDS fold current multiple-PD/PI awards and subprojects of multicomponent awards such as P01s and P50s into the new R35 award?
Investigators who receive an RPA will be required to relinquish their other NINDS funding. In the case of multiple-PD/PI awards, they will relinquish their financial interest in the award but will be expected to continue the collaboration with support from RPA. This transition may be made at the end of a current budget period. The budget of the R01, P01, P50, etc. would be appropriately adjusted to reflect the cessation of support from the multiple-PD/PI award whenever that occurs. Investigators supported by RPA are expected to continue as associated investigators of the continuing P01 or P50 and thus be expected to interact with the other subproject investigators and to have access to the P01 or P50 cores.
Q. Once I am awarded an R35, can I also receive a MPI NINDS R01 award?
No, MPI NINDS R01 applications will not be awarded to investigators with active R35 awards, unless it is one of the listed exceptions in the NOFO not subject to consolidation into the RPA, see RFA-NS-22-038.
Q. Will a change of PI/PI be allowed?
A permanent change of PD/PI will not be allowed, as RPA is intended to support the research program of a single independent investigator. A temporary change may be allowed with prior approval under circumstances such as sabbatical leave, medical conditions, disability or personal or family situations.
Q. What happens if the PD/PI becomes unable to carry out the duties as PD/PI or will be absent for more than 3 months at a time for any reason?
Standard NIH and NINDS policies will be applied.
Q. Will a change of grantee institution be allowed?
Standard NIH and NINDS policies will be applied, provided that the receiving institution agrees to all of the required terms of the RPA.
Q. What, if anything, will be different about the annual reporting required for RPA?
Annual reports will be required using the Research Performance Progress Report (RPPR). Additional NOFO specific requirements will be indicated in the Notice of Award.
Q. What changes will require prior approval?
There are no changes to standard NIH policy on prior approval requirements. Prior approval is needed for the following:
- A change in scope including but not limited to, a change from the approved used of vertebrate animals or involvement of human subjects, select agents or human embryonic stem cells
- Late notification of an initial no cost extension
- Change in status of the PD/PI or senior/key personnel named in the Notice of Award
- Change in grantee organization or organization status
Q. How will NINDS manage overlap with other grants that may be awarded after the initial RPA?
Changes in other support should be reported in the RPPR. The relationship between other support and work supported by RPA should be explained. NINDS will assess whether there is sufficient scientific and budgetary overlap to warrant adjustment of the RPA.
Q. Will carryover of an unobligated balance from one budget period to another be permitted?
Standard NIH and NINDS policies will be applied.
Q. How will NINDS handle changes in senior/key personnel on a RPA?
Senior/key personnel named in the notice of award may be replaced or eliminated from the budget with prior approval. Applicants should report such changes in their annual progress reports and should include plans for the utilization of the funds to support the overall effort of the laboratory. Persons not named in the notice of award can be replaced without prior approval.
Q. How will receipt of support from RPA effect the eligibility of co-PD/PIs to receive other grants?
Senior/key personnel other than the PD/PI may receive support from other awards, including other RPA awards on which they are also not the PD/PI. Independent investigators who receive any support from more than one NINDS grant are encouraged to consider consolidating their support by applying for a RPA application when they become eligible under future NOFOs.
Q. I have an NINDS R35 award. Can I apply for an NINDS grant supported by the BRAIN, ADRD, CounterACT and/or HEAL Initiatives?
Yes. R35 awardees can apply for funding opportunities supported by BRAIN, ADRD, CounterACT and/or HEAL funds earmarked to NINDS; however, you should contact your R35 Program Officer prior to submitting any new applications to NINDS. Please note that you are required to maintain 50% effort on your R35 award throughout its eight-year duration, and no reduction in effort below 50% will be approved to accommodate additional grant awards. Should you receive additional funding through one of the programs listed above, you will be expected to allocate a reasonable level of effort to that new activity. Investigators should also note that awards made through one of the programs listed above will be included in determining whether you are considered a “well-funded investigator” as it relates to NINDS’ Special Council Review Policy (see NOT-NS-24-060).
Q. I have an NINDS R35 award. Can I apply for an administrative supplement (aka revision)?
R35 awardees can apply for an administrative supplement, however it is expected that supplements will be limited to a single year and will not generally be made in excess of $100K Direct Costs (DC). The only exceptions to the policy outlined above is for supplements submitted in response to specific solicitations using BRAIN, ADRD, CounterACT and HEAL funds (see question above). Competitive supplement (aka revision)? Unless solicited via a specific NOFO, R35 awardees are not eligible to apply for competitive supplements.
Q. I have an NINDS R35 award. Can I participate in a new or renewal application for an NINDS-funded P01 or Center?
Yes, however P01/ Center activities must be supported using the funds awarded through your R35. No additional funds will be awarded to you (or your lab) to support salaries or research activities on the P01/Center grant. You ARE, however permitted to receive P01 or Center funds to lead a core (either administrative or scientific) as these are considered research resources that support the efforts of the P01/Center in totality.
Q. I have an NINDS R35 award. Can I continue to receive support from another PI’s grant as a co-investigator?
Yes, however it is expected that the total amount of support you receive serving in these capacities will not significantly exceed the amount held at the time you submitted your R35 application. Receipt of funds as a co-Investigator should not be used to circumvent the budget limits of the R35.
Resources
Research Program Award (R35 Clinical Trial Optional) (RFA-NS-22-038)
Anna Taylor and Bob Finkelstein. “An experiment in program-based funding.”
Inquiries/Contacts
Alisa Schaefer, Ph.D. | Program Officer, Division of Extramural Activities
alisa.schaefer@nih.gov
