Department of Health and Human Services
National Institutes of Health
National Institute of Neurological Disorders and Stroke
Table of Contents
(Skip)
- Organization Chart
- Appropriation Language
- Amounts Available for Obligation
- Budget Mechanism Table
- Major Changes in Budget Request
- Summary of Changes
- Budget Graphs
- Budget Authority by Activity
- Authorizing Legislation
- Appropriations History
- Justification of Budget Request
- Budget Authority by Object Class
- Salaries and Expenses
- Detail of Full-Time Equivalent Employment (FTE)
- Detail of Positions
Organization Chart
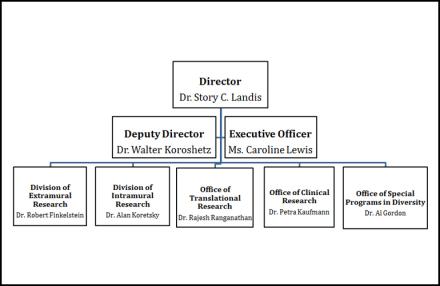
National Institutes of Health
National Institute of Neurological Disorders and Stroke
For carrying out section 301 and title IV of the PHS Act with respect to neurological disorders and stroke, $1,642,619,000.
Amounts Available for Obligation 1
(Dollars in Thousands)
| Source of Funding | F Y 2012 Actual |
F Y 2013 CR |
F Y 2014 PB |
|---|---|---|---|
|
Appropriation |
1,629,445 (3,080) |
1,636,319 0 |
1,642,619 0 |
| Subtotal, adjusted appropriation | 1,626,365 | 1,636,319 | 1,642,619 |
| Secretary's Transfer for Alzheimer disease (AD) | (1,072) | 0 | 0 |
| Secretary's Transfer for AIDS authorized by PL 112-74, Section 206 | (463) | 0 | 0 |
| Comparative Transfers to NLM for NCBI and Public Access | (1,486) | (1,925) | 0 |
| Subtotal, adjusted budget authority | 1,623,344 | 1,634,394 | 1,642,619 |
| Unobligated balance, start of year | 0 | 0 | 0 |
| Unobligated balance, end of year | 0 | 0 | 0 |
| Subtotal, adjusted budget authority | 1,623,344 | 1,634,394 | 1,642,619 |
| Unobligated balance lapsing | (44) | 0 | 0 |
| Total obligations | 1,623,300 | 1,634,394 | 1,642,619 |
1 Excludes the following amounts for reimbursable activities carried out by this account:
FY 2012 - $10,601 FY 2013 - $10,642 FY 2014 - $10,684
Budget Mechanism - Total 2
(Dollars in Thousands)
| MECHANISM | F Y 2012 Actual |
F Y 2013 CR |
F Y 2014 PB |
Change vs. F Y 2012 |
||||
|---|---|---|---|---|---|---|---|---|
| No. | Amount | No. | Amount | No. | Amount | No. | Amount | |
| Research Grants | ||||||||
| Research Projects: | ||||||||
| Noncompeting | 1,941 | $815,682 | 1,895 | $776,000 | 2,005 | $776,939 | 64 | -$38,743 |
| Administrative Supplements | (109) | 10,389 | (105) | 10,000 | (105) | 10,000 | -(4) | -389 |
| Competing: | ||||||||
| Renewal | 160 | 84,263 | 192 | 75,065 | 198 | 77,101 | 38 | -7,162 |
| New | 537 | 179,349 | 636 | 237,704 | 639 | 239,245 | 102 | 59,896 |
| Supplements | 2 | 419 | 0 | 0 | 0 | 0 | -2 | -419 |
| Subtotal, Competing | 699 | $264,031 | 828 | $312,769 | 837 | $316,346 | 138 | $52,315 |
| Subtotal, RPGs | 2,640 | $1,090,103 | 2,723 | $1,098,769 | 2,842 | $1,103,285 | 202 | $13,182 |
| SBIR/STTR | 90 | 41,723 | 96 | 44,658 | 101 | 46,996 | 11 | 5,273 |
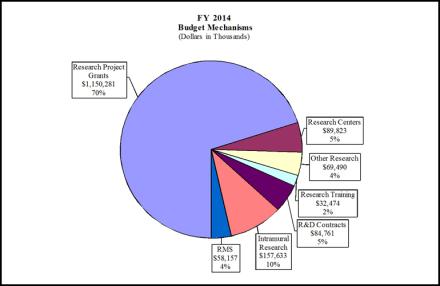
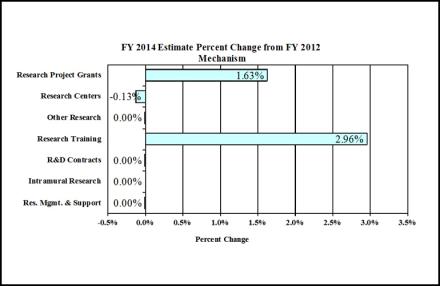
| Research Project Grants | 2,730 | $1,131,826 | 2,819 | $1,143,427 | 2,943 | $1,150,281 | 213 | $18,455 |
Research Centers: |
||||||||
| Specialized/Comprehensive | 81 | 88,371 | 86 | 88,258 | 81 | 88,258 | 0 | -113 |
| Clinical Research | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Biotechnology | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Comparative Medicine | 0 | 1,565 | 0 | 1,565 | 0 | 1,565 | 0 | 0 |
| Research Centers in Minority Institutions | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Research Centers | 81 | $89,936 | 86 | $89,823 | 81 | $89,823 | 0 | -$113 |
Other Research: |
||||||||
| Research Careers | 213 | 35,957 | 206 | 35,957 | 213 | 35,957 | 0 | 0 |
| Cancer Education | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cooperative Clinical Research | 78 | 15,880 | 55 | 15,880 | 78 | 15,880 | 0 | 0 |
| Biomedical Research Support | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Minority Biomedical Research Support | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 103 | 17,653 | 103 | 17,653 | 103 | 17,653 | 0 | 0 |
| Other Research | 394 | $69,491 | 364 | $69,490 | 394 | $69,490 | 0 | -$1 |
| Total Research Grants | 3,205 | $1,291,253 | 3,269 | $1,302,740 | 3,418 | $1,309,594 | 213 | $18,341 |
Ruth L. Kirschstein Training Awards: |
FTTPs |
FTTPs |
FTTPs |
|||||
| Individual Awards | 412 | 16,383 | 412 | 16,383 | 412 | 16,711 | 0 | 328 |
| Institutional Awards | 330 | 15,157 | 330 | 15,157 | 330 | 15,763 | 0 | 606 |
| Total Research Training | 742 | $31,540 | 742 | $31,540 | 742 | $32,474 | 0 | $934 |
Research & Development Contracts |
107 |
84,761 |
100 |
84,324 |
107 |
84,761 |
0 |
0 |
| SBIR/STTR (non-add) | (3) | (89) | (3) | (89) | (3) | (89) | (0) | +(0) |
FTE's |
FTE's |
FTE's |
FTE's |
|||||
| Intramural Research | 343 | 157,633 | 343 | 157,633 | 343 | 157,633 | 0 | 0 |
| Research Management and Support | 169 | 58,157 | 203 | 58,157 | 203 | 58,157 | 34 | 0 |
| Construction | 0 | 0 | 0 | 0 | ||||
| Buildings and Facilities | 0 | 0 | 0 | 0 | ||||
| Total, NINDS | 512 | $1,623,344 | 546 | $1,634,394 | 546 | $1,642,619 | 34 | $19,275 |
1 All items in italics and brackets are "non-adds."
Major Changes in the Fiscal Year 2014 President's Budget
Major changes by budget mechanism and/or budget activity detail are briefly described below. Note that there may be overlap between budget mechanisms and activity detail and these highlights will not sum to the total change for the FY 2014 President’s Budget for NINDS, which is $19.3 million more than the FY 2012 level, for a total of $1,642.6 million.
Research Project Grants (+$18.455 million, total $1,150.281 million): NINDS will support a total of 2,943 Research Project Grant (RPG) awards in FY 2014. Noncompeting RPGs will increase by 64 awards and decrease by $38.743 million. Competing RPGs will increase by 138 awards and $52.315 million. NIH budget policy for RPGs in FY 2014 discontinues inflationary allowances and average cost is assumed at the FY 2012 final allocation levels or lower.
Research Training (+$0.934 million, total $32.474 million): NIH will provide an increase of two percent over FY 2012 for stipend levels for pre-doctoral trainees. The Ruth L. Kirschstein National Research Service Award training program budget reflects a stipend increase to $42,000 for the entry level postdoctoral trainees and includes a four percent increase for each subsequent level of experience. The requested increase will help sustain the development of a highly qualified biomedical research workforce.
Summary of Changes
(Dollars in Thousands)
| F Y 2012 Enacted | $1,623,344 |
|---|---|
| F Y 2014 President's Budget | $1,642,619 |
| Net change | $19,275 |
| 2014 President's Budget |
Change from F Y 2012 |
|||
|---|---|---|---|---|
| CHANGES | FTE's | Budget Authority |
FTE's | Budget Authority |
| A. Built-in: 1. Intramural research: |
||||
| a. Annualization of January 2013 pay increase & benefits |
$54,067 | $139 | ||
| b. January F Y 2014 pay increase & benefits | 54,067 | 399 | ||
| c. One more day of pay | 54,067 | 205 | ||
| d. Differences attributable to change in FTE | 54,067 | 0 | ||
| e. Payment for centrally furnished services | 23,826 | 428 | ||
| f. Increased cost of laboratory supplies, materials, other expenses, and non-recurring costs |
79,740 | 173 | ||
| Subtotal | $1,344 | |||
2. Research Management and Support: |
||||
| a. Annualization of January 2013 pay increase & benefits |
$30,481 | $82 | ||
| b. January F Y 2014 pay increase & benefits |
30,481 | 225 | ||
| c. One more day of pay | 30,481 | 115 | ||
| d. Differences attributable to change in FTE | 30,481 | 0 | ||
| e. Payment for centrally furnished services | 12,033 | 217 | ||
| f. Increased cost of laboratory supplies, materials, other expenses, and non-recurring costs |
15,643 | 3 | ||
| Subtotal | $642 | |||
Subtotal, Built-in |
$1,986 | |||
| 2014 President's Budget |
Change from F Y 2012 |
|||
|---|---|---|---|---|
| CHANGES | No. | Amount | No. | Amount |
| B. Program: | ||||
| 1. Research Project Grants: | ||||
| a. Noncompeting | 2,005 | $786,939 | 64 | -$39,132 |
| b. Competing | 837 | 316,346 | 138 | 52,315 |
| c. SBIR/STTR | 101 | 46,996 | 11 | 5,273 |
| Total | 2,943 | $1,150,281 | 213 | $18,456 |
| 2. Research Centers | 81 | $89,823 | 0 | -$113 |
| 3. Other Research | 394 | 69,490 | 0 | -1 |
| 4. Research Training | 742 | 32,474 | 0 | 934 |
| 5. Research and Development Contracts | 107 | 84,761 | 0 | 0 |
| Subtotal, Extramural | $1,426,829 | $19,276 | ||
| FTE's | FTE's | |||
| 6. Intramural Research | 343 | $157,633 | 0 | -$1,344 |
| 7. Research Management and Support | 203 | 58,157 | 34 | -642 |
| 8. Construction | 0 | 0 | ||
| 9. Buildings and Facilities | 0 | 0 | ||
| Subtotal, Program | 546 | $1,642,619 | 34 | $17,290 |
| Total Changes | $19,275 | |||
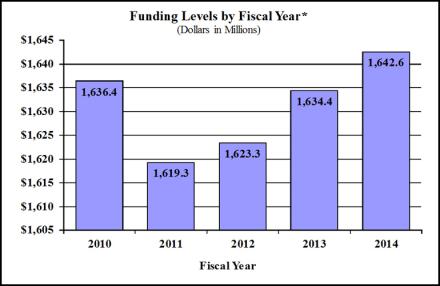
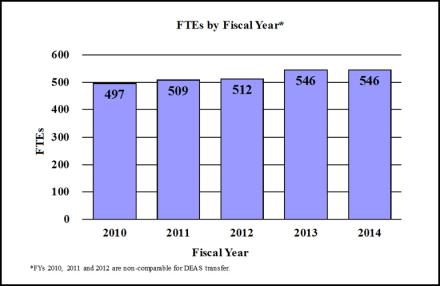
Budget Graphs
History of Budget Authority and FTE's:
Distribution by Mechanism:
Change by Selected Mechanisms:
Budget Authority by Activity
| F Y 2012 Actual |
F Y 2013 CR |
F Y 2014 PB |
Change vs. F Y 2012 |
|||||
|---|---|---|---|---|---|---|---|---|
| Extramural Research Detail: |
FTE's | Amount | FTE's | Amount | FTE's | Amount | FTE's | Amount |
| Channels, Synapses & Circuits | 133,313 | 134,360 | 135,139 | 1,826 | ||||
| Infrastructure, Training Programs, and Resources | 204,191 | 205,794 | 206,987 | 2,796 | ||||
| Neural Environment | 218,711 | 220,428 | 221,706 | 2,995 | ||||
| Neurodegeneration | 206,667 | 208,290 | 209,497 | 2,830 | ||||
| Neurogenetics | 201,649 | 203,232 | 204,411 | 2,762 | ||||
| Repair & Plasticity | 124,326 | 125,302 | 126,029 | 1,703 | ||||
| Systems & Cognitive Neuroscience | 234,314 | 236,153 | 237,522 | 3,208 | ||||
| Translational Research | 84,383 | 85,045 | 85,538 | 1,155 | ||||
| Subtotal, Extramural | $1,407,554 | $1,418,604 | $1,426,829 | $19,275 | ||||
Intramural Research |
343 | $157,633 | 343 | $157,633 | 343 | $157,633 | 0 | ($0) |
Research Management & Support |
169 | $58,157 | 203 | $58,157 | 203 | $58,157 | 34 | ($0) |
TOTAL |
512 | $1,623,344 | 546 | $1,634,394 | 546 | $1,642,619 | 34 | $19,275 |
1 Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
2 Includes Transfers and Comparable Adjustments as detailed in the "Amounts Available for Obligation" table.
Authorizing Legislation
| PHS Act/ Other Citation |
U.S. Code Citation |
2012 Amount Authorized |
F Y 2012 Enacted |
2013 Amount Authorized |
F Y 2013 PB |
|
|---|---|---|---|---|---|---|
| Research and Investigation | Section 301 | 42§241 | Indefinite | $1,634,394,000 | Indefinite | $1,642,619,000 |
| National Institute of Neurological Disorders and Stroke |
Section 401(a) | 42§281 | Indefinite | Indefinite | ||
| Total, Budget Authority | $1,634,394,000 | $1,642,619,000 |
Appropriations History
| Fiscal Year | Budget Estimate to Congress | House Allowance | Senate Allowance | Appropriation |
|---|---|---|---|---|
| 2005 | $1,545,623,000 | $1,545,623,000 | $1,569,100,000 | $1,539,448,000 |
| Rescission | ($12,675,000) | |||
| 2006 | $1,550,260,000 | $1,550,260,000 | $1,591,924,000 | $1,550,260,000 |
| Rescission | ($1,503,000) | |||
| 2007 | $1,524,750,000 | $1,524,750,000 | $1,537,703,000 | $1,534,757,000 |
| Rescission | - | |||
| 2008 | $1,537,019,000 | $1,559,106,000 | $1,573,268,000 | $1,571,353,000 |
| Rescission | ($27,452,000) | |||
| 2009 | $1,545,397,000 | $1,598,521,000 | $1,588,405,000 | $1,593,344,000 |
| Rescission | - | |||
| Supplemental | $8,212,000 | |||
| 2010 | $1,612,745,000 | $1,650,253,000 | $1,620,494,000 | $1,636,371,000 |
| Rescission | - | |||
| 2011 | $1,681,333,000 | - | $1,678,696,000 | $1,636,371,000 |
| Rescission | ($14,368,312) | |||
| 2012 | $1,664,253,000 | $1,664,253,000 | $1,603,741,000 | $1,629,445,000 |
| Rescission | ($3,079,651) | |||
| 2013 | $1,624,707,000 | - | $1,629,631,000 | - |
| Rescission | - | |||
| 2014 | $1,624,619,000 | - | - | - |
Justification of Budget Request
National Institute of Neurological Disorders and Stroke
Authorizing Legislation: Section 301 and Title IV of the Public Health Service Act, as amended.
Budget Authority (BA):
| F Y 2012 Actual |
F Y 2013 CR |
F Y 2014 President's Budget |
F Y 2014 +/- F Y 2012 |
|
|---|---|---|---|---|
| BA | $1,623,344,000 | $1,634,394,000 | $1,642,619,000 | +$19,275,000 |
| FTE | 512 | 546 | 546 | +34 |
Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
Director's Overview
The mission of the National Institute of Neurological Disease and Stroke (NINDS) is to reduce the burden of neurological disorders through research. Hundreds of disorders affect the brain and spinal cord, inflicting an enormous toll of suffering, disability, and death. Better prevention and care for these diseases have improved life for many, but too often the best medical options only partly suppress symptoms without halting the underlying disease, and they cannot repair damage to the brain. The variety of diseases, together with the complexity and inaccessibility of the brain, challenge the current limits of medicine. However, progress on many frontiers of science, engineering, and medicine is advancing the fight against neurological disorders through the combined efforts of NIH and the private sector.
Academia and industry agree that basic research on the brain in health and disease is the foundation for progress. The potential for payoff from basic research is too far removed and unpredictable to attract substantial private investment. NIH investigator-initiated programs that engage the wisdom and ingenuity of the scientific marketplace of ideas are especially suited to basic science. A wealth of new technologies is driving neuroscience— mapping the intricacies of brain structure; activating brain circuits with light pulses; monitoring activity in thinking human brains; and sequencing whole genomes sequencing, among many others capabilities. The following illustrate recent benefits and future promise of NIH-supported basic research:
- Twenty years ago there were no effective drugs for multiple sclerosis. Basic research on the immune system and the brain has led to eight drugs that are now available, with the first oral drugs approved this year and several more drugs in advanced clinical testing.
- Knowledge of brain circuits is the foundation for deep brain stimulation (DBS) therapy. Clinical trials this year confirmed the long-term benefit of DBS for Parkinson’s disease and revealed the promise for epilepsy and other diseases. The NIH Blueprint Connectome Project and the new Brain Research through Application of Innovative Neurotechnologies Project develop and apply cutting-edge technologies to revolutionize anatomical and functional understanding of brain circuitry.
- This year’s Nobel Prize to a long-term NINDS grantee recognized the impact of basic research on a class of receptors, G-protein coupled receptors, through which about half of all drugs work. These receptors sense neurotransmitters, and other chemical signals.
- Basic researchers have isolated stem cells and learned to control their fate. Stem cell therapy clinical trials have begun in diseases as diverse as spinal cord injury, amyotrophic lateral sclerosis, and Pelizaeus-Merzbacher disease, with NIH and private support.
- Researchers sequenced the entire genome of twins with a rare form of dystonia and quickly discovered that a known drug would dramatically improve their lives. Though the impact is rarely so rapid, gene discoveries have provided diagnostics and a rational way forward to develop therapies for many other previously baffling gene disorders.
For decades, NINDS research has translated scientific discoveries into better treatments. Paradoxically, despite increasing opportunities from basic science, pharmaceutical companies are reducing investment in drug development for neurological disorders because of the challenges brain diseases present. The following illustrate the Institute’s array of programs to take advantage of the increased opportunities and address opportunities for translational research:
- In its first decade, projects from the milestone-driven Cooperative Program in Translational Research have filed eight Investigative New Drug applications with the FDA; three are now in NINDS clinical trials and others are moving forward in the private sector.
- The goal of the NINDS-led NIH Blueprint Neurotherapeutics Network Grand Challenge is to develop novel drugs that will transform treatment of nervous system disorders, from common diseases like Alzheimer’s to rare disorders such as familial dysautonomia.
- The Neural Interfaces Program pioneered devices that restore functions of the nervous system lost from disease or injury. This year, neural interface technology enabled paralyzed patients to control a robotic arm with signals detected directly from their brains.
- The Network for Excellence in Neuroscience Clinical Trials (NeuroNEXT) is speeding early phase clinical testing of novel therapies. The network’s first projects are clinical studies of spinal muscular atrophy biomarkers and a clinical trial on secondary progressive multiple sclerosis, with plans for clinical trials of two therapies emerging from the NCATS Therapeutics for Rare and Neglected Disease Program.
- The Parkinson’s Disease Biomarkers Program will expedite therapy development by developing measures of disease progression and response to treatment.
- Induced Pluripotent Stem Cell Consortia (iPSC) are developing patient-derived stem cells for amyotrophic lateral sclerosis, Parkinson’s, and Huntington’s disease. This year the Consortium demonstrated the value of iPSCs for rapid Parkinson’s disease drug screening.
- The Anticonvulsant Screening Program, which has contributed to eight drugs on the market for epilepsy, will now focus on drugs to prevent epilepsy and for treatment resistant epilepsy, which are also priorities for the Epilepsy Centers without Walls program for 2014.
- An initiative on the blood brain barrier, which protects the brain but is an obstacle for many potential drugs, will catalyze public and private therapy development for many diseases.
- New initiatives on stroke prevention and treatment build on past success and reflect an extensive planning process, which engaged the public and experts from several disciplines.
- The Neurological Emergency Treatment Trials network completed its first multi-site clinical trial, demonstrating the effectiveness of a treatment for continuous seizures and the efficiency of this network. Trials are underway for stroke and traumatic brain injury.
- In 2014, a large observational study will address major obstacles that have confounded development of treatment for traumatic brain injury (TBI).
A vigorous and innovative scientific workforce is essential to continued progress against neurological disorders. This priority is evident throughout NINDS programs and policies, through specific programs, such as the Neuroscience Scholars Program, and through participation in trans-NIH programs, such as the Pathway to Independence Awards. To recruit and sustain a talented and diverse workforce, students and new investigators must see that intelligence and creativity, honed through years of intensive training and apprentice research, will yield a fair chance for obtaining independent research support. Toward this end, NINDS’ highest program priority is the funding success rate for investigator-initiated research, with particular attention to new investigators. In the past year, NINDS grantees received a Nobel Prize for basic science, a MacArthur Award for innovative clinical work, and several top ratings in the highly competitive NIH Transformative R01 program. These awards reflect the excitement and innovation of neuroscience, which bodes well for the future.
Overall Budget Policy:
The FY 2014 President's Budget request is $1,642.619 million, an increase of $19.275 million or 1.3 percent, above the FY 2012 Actual level. NINDS emphasizes investigator-initiated research throughout its programs, but also targets solicitations to address unmet, mission-critical scientific opportunities and public health needs. In recent years, the Institute raised funding success rates for investigator initiated research, with special attention to new investigators, and launched several new targeted programs. To accomplish this, NINDS rigorously evaluates programs, in consultation with the National Advisory Neurological Disorders and Stroke Advisory Council and outside experts, and closes programs that no longer warrant investment, because goals have been met, science has advanced, or programs have not proven effective. Clinical trials are inherently expensive, but scheduled interim analyses of progress have allowed the Institute to terminate large clinical trials early if the clinical question has been answered, saving millions of dollars. Similarly, NINDS monitors milestones in preclinical therapy development projects, shifting funds to the best current opportunities. NINDS evaluates the mission relevance of all requests to submit applications for large investigator-initiated projects and sunsets multi-investigator program project grants after one renewal period. Across all programs, NINDS has enhanced attention to rigor and reproducibility of research, especially for studies that justify major investments in preclinical therapy development or clinical trials. In 2012 NINDS convened NIH staff, scientists, patient advocates, journal editors, and other stakeholders to discuss this issue, and the Institute is implementing recommendations, which were published in a highly respected scientific journal 1. Funds are included in R&D contract to support trans-NIH initiatives, such as the Basic Behavioral and Social Sciences Opportunity Network (OppNet).
1Nature 490:187-91, 2012
Program Descriptions and Accomplishments
Channels, Synapses, and Circuits:
Ion channels, synapses, and circuits of interacting nerve cells are fundamental components of the nervous system. Ion channels control electrical currents in cells. Synapses are the connections by which nerve cells influence the activity of other nerve cells. Circuits formed by networks of interconnected nerve cells carry out the higher functions of the brain. NINDS supports research on how channels, synapses, and circuits operate in the healthy nervous system in the adult and developing brain and on neurological disorders in which these elements play a major role. The program encompasses basic, translational, and clinical research. Topics of basic research include neural circuit analysis, synaptic transmission, synaptic plasticity, and structural analysis of neuronal membrane proteins. Epilepsy, a disease in which channels, synapses, and brain circuits are perturbed, is a common disorder, affecting nearly one percent of the U.S. population 2. The Institute continues its longstanding and comprehensive research program, from basic laboratory studies through multi-site clinical trials, that has contributed to many advances in epilepsy treatment. Since 2001, the Epilepsy Benchmarks process has brought NINDS, the research community, and non-governmental organizations together to establish milestones and monitor progress toward the goal of "no seizures, no side effects." In accord with those goals, NINDS emphasizes research on identifying the causes of epilepsy, preventing the epilepsies and their progression, developing new strategies for treatment resistant epilepsy, and addressing co-morbidities of epilepsy, including Sudden Unexplained Death in Epilepsy (SUDEP).
Budget Policy: The FY 2014 President's Budget estimate is $135.139 million, an increase of $1.826 million above the FY 2012 Actual level. In 2014, NINDS will continue to balance investigator-initiated research and research targeted to specific mission priorities, including projects funded through the Institute's translational research and clinical trials programs. In 2014, NINDS, working with NIMH and the broad neuroscience community, will launch the Brain Research through Application of Innovative Neurotechnologies Project, which builds on recent advances in science and technology to develop methods to understand in detail how large circuits of nerve cells carry out the higher functions of the brain, with implications for a wide range of diseases. In April 2013, a major Curing Epilepsy Conference will continue the Epilepsy Benchmarks process, which for more than a decade has brought the NIH, the research community, and patient advocates together to establish research priorities and monitor progress. The Epilepsy Centers without Walls program is continuing. Each center brings together the best multidisciplinary team of investigators, regardless of geographic locations, to focus for multiple years on a specific Benchmarks priority that is not easily addressed through regular grant mechanisms. The Center on epilepsy genetics and genomics, for example, has through a team effort identified several new mutations in known epilepsy genes and also identified previously unrecognized genes that contribute to infantile spasms and Lennox-Gastaut syndrome. Other centers and planning grants to develop centers are addressing SUDEP (Sudden Unexplained Death in Epilepsy) and disease modification and prevention of epilepsy. NINDS and NHLBI are also collaborating to expand the CDC's Sudden Unexpected Infant Death (SUID) Case Registry to include SUDEP and Sudden Cardiac Death in individuals up to age 24 in 15 states.
2Neurology 68:326-37, 2007
Program Portrait: Brain Research through Application of Innovative Neurotechnologies (NIH BRAIN)
FY 2012 Level: $0.0 million
FY 2014 Level: $7.5 million
Change: + $7.5 million
People perceive, think, and act through networks of brain cells that influence one another’s activity in precise ways, like electronic circuits but much more complex. Knowledge of how brain circuits work has immense potential for understanding and alleviating disease. For example, deep brain stimulation therapy can compensate for malfunctioning brain circuits in Parkinson’s disease, dystonia, and other disorders. However, the inadequacy of methods to monitor complete, functioning circuits of brain cells limits research to understand brain circuits. Existing methods either detect activity of too few cells at a time, are too slow to follow the millisecond speed changes in brain activity, or assess the summed activity of entire regions of the brain containing millions of cells.
Through the BRAIN, NINDS, in partnership with the National Institute of Mental Health (NIMH), the Defense Advanced Research Projects Agency (DARPA), and the Office of Science and Technology Policy, will increase dramatically our capacity to monitor the activity of nerve cells and understand brain circuits. The ambitious goal is to increase our capabilities to monitor cells by at least three orders of magnitude over the next five years, with the target of recording from a million cells at once. The ultimate goal is to compensate for malfunction in brain circuits when vision, hearing, movement, control, touch, or even memory is lost. Although the technical challenges are formidable, a convergence of developments in neuroscience with advances in nanotechnology, physics, optics, biochemistry, computer science, genetic engineering, and several other disciplines, now makes the Project feasible. In addition to mapping the “neural code” by which brain circuits operate, the Project will stimulate development on several technological frontiers, which is likely to yield unanticipated benefits, just as did the Human Genome Project and NASA’s Apollo Project. Not the least of the challenges is developing computational tools to make sense of the simultaneous, high time resolution monitoring of a million nerve cells, which will have payoffs for other massive data applications from health data to genomics.
Infrastructure, Training programs, and Resources:
To enhance the effectiveness of all NINDS extramural programs, the Institute supports common infrastructure and programs related to clinical research and clinical trials, training and career development, workforce diversity, and health disparities. The Office of Clinical Research (OCR) is the focal point for large clinical trials and epidemiological studies at NINDS, working closely with disease-focused programs in the Institute. OCR programs that support clinical trials have significantly increased the efficiency and effectiveness of these inherently expensive activities by monitoring progress with milestones that allow early termination when appropriate and by developing multi-site clinical networks that provide common resources and economies of scale. The Network for Excellence in Neuroscience Clinical Trials (NeuroNEXT), established in 2012, is a multi-site network for early phase clinical trials of novel therapies for neurological disorders and related studies. This network provides remarkable efficiencies such as a single Institutional Review Board and master agreements among clinical sites, substantially reducing the time and cost required to launch new trials. NeuroNEXT also protects the intellectual property of investigators, a key factor that will enable NINDS to partner with industry, foundations, or academia to test the most promising interventions. Another network, the multi-site Neurological Emergency Treatment Trials Network (NETT), brings together experts from neurology, neurosurgery, emergency medicine, and other medical disciplines to conduct clinical trials for stroke, traumatic brain injury, and other neurological emergencies. This year NETT completed, ahead of schedule and under budget, a clinical trial that demonstrated the effectiveness of an emergency treatment for status epilepticus, potentially fatal continuous seizures. The OCR Common Data Elements program works with the research and patient advocacy communities to develop standards that enable comparison and sharing of clinical data across studies. The Common Data Elements for neuromuscular diseases, multiple sclerosis, Huntington's disease, traumatic brain injury, epilepsy, Friedreich's ataxia, frontotemporal dementia, Parkinson's disease, spinal cord injury, and stroke are now available. Following the advice of strategic planning panels on health disparities and on workforce diversity, the Institute has integrated health disparities research within OCR. Similarly, diversity activities are now integrated with all NINDS training programs through the renamed Office of Training, Career Development, and Workforce Diversity. NINDS also continues to support infrastructure programs through the Office of Special Programs in Diversity.
Budget Policy: The FY 2014 President's Budget estimate is $206.987 million, an increase of $2.796 million above the FY 2012 Actual level. In 2012, NINDS funded the data coordinating center, clinical coordinating center, and 25 clinical sites across the U.S. for the NeuroNEXT clinical network and began the first clinical study, on developing biomarkers to accelerate therapy development for spinal muscular atrophy (SMA). NINDS also issued solicitations targeted at academic investigators, foundations, small businesses, and industry for the first clinical trials to be conducted in the network, to begin in 2013, and these solicitations will be open for new submissions in 2014. This level will also support the continuation of the NETT program in 2014. The network completed its first major clinical trial ahead of schedule, demonstrating the effectiveness of a treatment for emergency seizures. Major clinical trials for TBI and stroke are underway. The CDE program is emphasizing implementation, which includes working with investigators to integrate CDEs into data platforms, communicating the value of these data standards, and training and support in their use, as well as quality improvement and updates in response to scientific progress. A full range of NINDS programs in training and career development, are also continuing, including individual and institutional grants at the graduate, post-doctoral, and career development levels, including programs targeted to special needs, such as promoting diversity and enabling neurosurgeons to accommodate research preparation into their demanding training requirements. In FY2013 the Office of Special Programs in Diversity is funding a continuation of the Specialized Neuroscience Research Program, incorporating recommendations from the NINDS Workforce Diversity Strategic Planning Advisory Panel.
Neural Environment:
Non-nerve cells, called glial cells, outnumber nerve cells in the brain. These cells, together with specialized blood vessels and immune cells, maintain the local environment around nerve cells, fight infections, and control which molecules get into the brain from the circulating blood through the blood-brain barrier. NINDS supports basic, translational, and clinical research on the normal neural environment and on diseases in which its disruption plays a major role. Neurological disorders can result when non-neuronal cells are compromised, as in multiple sclerosis; when these cells themselves become aggressors, as in brain tumors; when viruses, bacteria, or parasites infect the nervous system, as in NeuroAIDS; or when the blood supply to brain cells is compromised, as in stroke. In recent years, there has been a sea change in understanding of glial cells. Once regarded as merely supporting cells, they are now recognized to be active in brain functions as diverse as the development of nerve cell connections, the transition from acute to chronic pain, and memory. Basic glial cell biology continues to be a high priority. Stroke is the most common disorder in which the neural environment is a central issue. In 2012, the Institute completed an extensive and inclusive two- phase planning process for stroke research. First, in 2011, the Stroke Progress Review Group (SPRG) conducted a final review of the stroke research landscape, ten years after the first SPRG established research priorities. In sixteen working group reports, posted in January 2012, more than 140 experts and public representatives evaluated progress and unmet challenges, highlighting priorities in each aspect of stroke research. Then, NINDS solicited proposals for actionable stroke priorities from SPRG members, the research community, professional societies, and the public. A working group reported to the NANDS Council in September 2012 after intensive assessment of which proposed initiates could most effectively advance stroke research and care over the next five years.
Budget Policy: The FY 2014 President's Budget estimate is $221.706 million, an increase of $2.995 million above the FY 2012 Actual level. NINDS will continue to balance research targeted to specific priorities and investigator-initiated research, including research through the Institute's translational research and clinical trials programs. For 2014, a series of NINDS initiatives will address priorities in stroke research, including both prevention and treatment. These priorities reflect an extensive and inclusive stroke planning process that reported to the NANDS Council in 2012. The Institute is continuing to collaborate with the National Cancer Institute (NCI) in support of Specialized Programs of Research Excellence (SPORE) center grants that support highly interactive translational research to improve prevention, early detection, diagnosis, and treatment of brain tumor or other nervous system tumors. The Institute supports research on HIV/AIDS related to the Institute's mission, with the current emphasis on the brain reservoir of the HIV virus, which persists despite the dramatically improved drug treatment for the disease. These efforts complement the increased emphasis on NeuroHIV in the NINDS Intramural Research Program.
Program Portrait: Stroke Clinical Trials Network
FY 2012 Level: $0.0 million
FY 2014 Level: $13.0 million
Change: + $13.0 million
According to the American Heart Association, stroke research contributed to a reduction in age-adjusted stroke death rate of 34.8 percent from 1998 to 2008, and the actual number of stroke deaths declined by 19.4 percent1. However, further progress is essential to continue the reduction in stroke burden because of the aging population. To ensure progress, an intensive NINDS stroke planning process identified priorities in stroke prevention, treatment, and recovery research. Workgroups in each of these areas (Prevention, Treatment, and Recovery) highlighted the need for stroke clinical trials network infrastructure to accelerate progress. In 2014, NINDS will develop a clinical trials network large and flexible enough to plan and execute stroke research in prevention, treatment, and recovery.
Clinical trials are time consuming and expensive, often requiring 10 years or more to complete. In the traditional model, a consortium of clinical sites is created for each new multi-center trial. This leads to duplication of infrastructure resources and lost time because of common start-up activities that are repeated for each trial. A single, large, flexible network will enhance efficiency and effectiveness compared with this traditional model, and is also more effective and efficient than separate networks focused on prevention, treatment, and recovery. A flexible network could accommodate the best stroke proposals as the opportunities arise in any of the three areas, which would ensure the network is productively engaged. The functions of Clinical and Data Coordinating Centers are not specific to prevention, treatment or recovery projects. Stroke expertise in prevention, treatment and recovery tend to be concentrated in the same centers, and NINDS would reach out to other sites if needed to carry out specialized projects. The new network will build on lessons learned from the SPOTRIAS (Specialized Programs of Translational Research in Stroke) consortium evaluation, which was presented at the NANDS Council in September, 2012, and learn from the strengths of other NINDS-supported clinical trial programs. The network will, for example, emphasize multidisciplinary collaboration within and among sites, enhance efficiency through a central IRB (Institutional Review Board) and other centralized contract services through the Clinical Coordinating Center and Data Coordinating Center, include a training component at clinical sites, help develop and execute projects proposed from a range of sources, from both outside and inside the Network, and have the capability to perform trials with a private partner.
1Circulation 125:e12-230, 2012
Neurodegeneration:
The Neurodegeneration program focuses on adult onset neurodegenerative diseases, that is, diseases in which brain cells progressively die. Alzheimer's disease, amyotrophic lateral sclerosis (ALS), frontotemporal dementias (FTD), Huntington's disease, Parkinson's disease, and vascular cognitive impairment are among the neurodegenerative diseases that affect adults. These diseases present an increasing human and economic challenge to the U.S. as our population ages. Research includes studies of disease mechanisms that identify potential therapeutic targets; animal model development; preclinical research on drug, gene, cell, and device therapies; and, clinical trials that test the safety and efficacy of new therapies. A major theme of this program is the recognition that shared mechanisms contribute to multiple neurodegenerative diseases, and thus may allow shared therapeutic strategies. Common disease mechanisms include perturbations of cells’ recycling machinery, protein mis-folding and aggregation, transmission of toxic proteins between nerve cells, and mitochondrial dysfunction. NINDS IPSC (Induced Pluripotent Stem Cell) Consortia, which are developing induced pluripotent stem cells from patients with ALS, Huntington’s, and Parkinson’s disease, showed this year the value of IPSCs for drug screening, as well as studies of disease mechanism. Studies of genetic and environmental influences, and their interaction, continue to be important aspects of the Program, including, for example, recent discovery of gene defects that contribute to both ALS and FTD. In some cases, gene mutations directly cause Alzheimer’s, Parkinson’s, and ALS, but in others, gene mutations play a subtle role in determining susceptibility; gene discoveries from inherited neurodegenerative diseases provide essential clues about underlying mechanisms for both. In addition to supporting the discovery of genetic factors, NINDS has supported the development of technology that will improve the efficiency of screening for known genetic factors. The importance of vascular factors in dementia is also increasingly recognized, both because of the public health impact of this common disorder and because commonalities and overlap between vascular dementias and Alzheimer’s disease have become apparent at every level of analysis, from molecular mechanisms to epidemiological risk factors.
Budget Policy: The FY 2014 President's Budget estimate is $209.497, an increase of $2.830 million above the FY 2012 Actual level. NINDS neurodegeneration research will continue to balance investigator-initiated research and solicited research, including projects funded through the Institute's translational research and clinical trials programs. The Morris K. Udall Parkinson's Disease Centers of Excellence program is continuing, including a strong emphasis on translational research. The ongoing Parkinson's Disease Biomarkers Program is developing and validating biomarkers, which are objectively measureable indicators of the disease process and drug actions that can accelerate the development of treatments. The Institute is also supporting development of biomarkers for pre-manifest Huntington's disease through ancillary investigations coordinated with the long term PREDICT-HD observational study. NINDS support for induced pluripotent stem cells (iPSCs) research and development for neurodegenerative diseases is making these powerful research and drug development tools widely available to the research community. In 2013, NINDS will lead a scientific conference on non-Alzheimer's dementias as part of the National Alzheimer's Project Act (NAPA) planning process that will guide further research in this area.
Neurogenetics:
Gene defects cause hundreds of diseases that affect the nervous system. Neurogenetic disorders include the ataxias, Down syndrome, dystonias, fragile X syndrome, lysosomal storage diseases, muscular dystrophies, peripheral neuropathies, Rett syndrome, spinal muscular atrophy, Tourette syndrome, and tuberous sclerosis, among many others. The Neurogenetics program supports research to identify gene defects that cause neurological disorders, on the molecular mechanisms through which genes act, and on the development of genetic animal and cell models of human disease. The Program also promotes the preclinical development and clinical testing of treatments for neurogenetic disorders. The identification of more than 200 genes related to neurological disorders has led to diagnostics that have made a major difference for families. These diagnostic tools often enable physicians to identify a disease within days and thereby short-cut or even eliminate the diagnostic odyssey of years of consultation with experts that many families confronted in the past. Gene findings also lead to animal models and rational strategies that revolutionize the search for therapies. In the last few years, several interventions have shown considerable promise in NINDS-supported laboratory studies and are moving into early clinical testing through NIH and private sector clinical trials for muscular dystrophies, Batten disease, fragile X syndrome, and inherited demyelinating disorders, among several other diseases. Rapid developments in gene sequencing technology are already accelerating research and, in a few cases, whole genome studies have even had an immediate impact on treating patients. Exploiting these opportunities is a high priority.
Budget Policy: The FY 2014 President's Budget estimate is $204.411 million, an increase of $2.762 million above the FY 2012 Actual level. NINDS will continue to support investigator-initiated grants and targeted activities in neurogenetics, including projects funded through the Institute's translational research and clinical trials programs. The Institute is providing substantial support for the application of whole genome sequencing and other "next generation" genomics methods to neurological disorders. In 2014, the Institute is continuing its support for the Paul D. Wellstone Muscular Dystrophy Cooperative Research Centers, which have a strong translational research component, and for the Autism Centers for Excellence; both are trans-NIH programs. NINDS is also participating in a new NICHD led solicitation for Centers of Collaborative Research in Fragile X syndromes. The Institute also works closely with the NIH Office of Rare Disease Research in supporting and providing disease specific expertise to nervous system disease related consortia within the Rare Diseases Clinical Research Network. NINDS-supported resources for neurogenetics research that enhance the efficiency and effectiveness of research include the NINDS Human Genetics Repository, which fosters sharing of clinically well characterized genetic material and cell lines among investigators.
Repair and Plasticity:
NINDS research on traumatic brain injury (TBI) and spinal cord injury covers the full spectrum from understanding the mechanisms of immediate damage and delayed damage that continues in the hours or even days after initial injury, through development of interventions in animal models and clinical testing of treatments to minimize or repair damage. Diagnostics and biomarkers are also a high priority, especially because of concerns that mild TBI from sports concussions or blast injury can lead to cognitive deficits or predispose to problems in later life. Stimulated by the high rate of traumatic brain injury among U.S. military personnel, NINDS has worked with other agencies, including the Departments of Defense and Veterans Affairs, on several trans-agency collaborative scientific workshops, on a joint NIH-DOD Federal Interagency Traumatic Brain Injury Research (FITBIR) database, and on the development with the research community of Common Data Elements (CDEs) for clinical research on TBI and spinal cord injury that will foster comparison across clinical studies and sharing of data. NINDS has also long supported research on stem cells, on the factors that stimulate and restrain regeneration of nerve fibers, and, more generally, on understanding and exploiting the inherent plasticity of the nervous system to repair damage from trauma or disease. As a result of extensive basic and preclinical research, clinical testing has begun with NIH and private sector support for cell and regenerative therapies for spinal cord injury, ALS, stroke, Pelizaeus-Merzbacher disease, and other disorders, though the obstacles for success are still formidable. Devices that interface with the nervous system offer another strategy for restoring nervous system functions lost to injury or disease. For more than thirty years, the NINDS Neural Interfaces Program has pioneered research on such devices. This year, for example, research from this program demonstrated that paralyzed patients could control a robotic arm with signals detected directly from their brains. Deep brain stimulation (DBS) is another area of current emphasis, including for example, improvement of electrodes, accuracy of electrode placement in the brain, and programming of stimulation devices, as well as continued studies to understand the mechanisms by which DBS provides benefits.
Budget Policy: The FY 2014 President's Budget estimate is $126.029 million, an increase of $1.703 million above the FY 2012 Actual level. NINDS continues to balance investigator-initiated research and solicitations, including projects funded through the Institute's translational research and clinical trials programs. A major clinical trial of progesterone as an emergency treatment for TBI is underway through the Neurological Emergency Treatment Trials clinical network. NINDS will also support observational studies of 5,000 adults and children with TBI that will improve the evidence base for care and address major obstacles that have hindered the development of better therapies for TBI. These studies build on the development of TBI Common Data Elements with the research community and take advantage of the NIH-Department of Defense led Federal Interagency TBI Informatics System (FITBIR) database to encourage sharing of data, including coordination with studies in Canada and the European community. In 2013 the Neural Interfaces Program will continue to solicit and support projects to translate advanced neural prosthetics and other devices up to and through "first in human" clinical demonstrations. NINDS is also working closely with the Foundation for NIH (fNIH) on TBI related research through a new fNIH Sports and Heath Research Program. The fNIH created this program with a major donation from the National Football League, but the research has public health relevance far beyond professional football. The initiatives will begin with a focus on Chronic Traumatic Encephalopathy (CTE), a neurodegenerative condition that results from repeated mild TBI.
Program Portrait: Traumatic Brain Injury (TBI) Research Initiative
FY 2012 Level: $0.0 million
FY 2014 Level: $10.0 million
Change: + $10.0 million
TBI is the leading cause of death and disability in children and young adults, a frequent problem among the elderly, and a major concern for the military and veterans. In 2014, NINDS will support a prospective, observational, multi-center study of 5,000 adults and children with TBI. Complementing this investigation, a study of 1,000 children will specifically evaluate the effectiveness of six major critical care guidelines for severe, pediatric TBI that are based on expert opinion rather than compelling experimental evidence. These studies will provide an evidence base for better diagnosis and treatment of TBI and address two major explanations for why more than 30 major clinical trials of interventions for TBI failed to demonstrate improved outcomes. First, current TBI classification systems are not adequate to deal with the heterogeneity of TBI, which can cause different types of damage and affect different parts of brain. That is, TBI patients who are now grouped together in clinical trials may respond differently to an intervention. Second, variations in TBI care and outcomes among different medical centers confound efforts to assess interventions in clinical trials. In addition, observational studies will advance diagnostics to determine who has sustained a mild TBI and when the brain has recovered, identification of genetic and environmental factors that affect the outcomes from TBI, and determination of age-related differences in mechanisms and outcomes.
NINDS has laid extensive groundwork for these studies. A unique opportunity for coordination with the European Union and the Canadian Institute of Health Research will allow comparison across 11,000 patients, enhancing the statistical power to detect important differences. The NINDS TBI Common Data Elements program brought the research community together to agree upon standards for data collection that will allow meaningful comparison across studies in the U.S. and internationally. The Department of Defense and NIH-led Federal Interagency TBI Informatics System (FITBIR) provides a common database for sharing of information among all qualified researchers. Finally, with funds from the American Recovery and Reinvestment Act, NINDS supported a pilot study of 600 TBI patients across the spectrum of severity, which will prepare researchers to move efficiently into a major study.
Systems and Cognitive Neuroscience:
Systems of interconnected nerve circuits in the brain, spinal cord, and body control learning, memory, attention, language, thinking, emotion, movement, the sleep-wake cycle, pain perception, and other complex behaviors. NINDS supports research on how systems of nerve cells carry out these functions and on counteracting the disruptive effects of neurological disorders on neural circuits. Stroke, brain trauma, and neurodegenerative diseases are among the disorders that affect cognition and other complex behaviors. Chronic pain disorders, including migraine and other headaches, are very prevalent and thus high priority areas of emphasis in this program. NINDS is the largest NIH supporter of research on pain. Last year, the NIH Director designated NINDS as the lead NIH institute for pain research. To enhance coordination of pain research, NINDS has newly dedicated scientific staff to coordinating pain research through the NIH Pain Consortium and with the wider Federal and private sector communities through the Interagency Pain Research Coordinating Committee (IPRCC). Ongoing activities of the IPRCC include an intensive assessment of advances in pain research and ongoing pain research across agencies. To increase the number of pain investigators, a key requirement for the future, NINDS has also increased support for career development (K) awards in pain research.
Budget Policy: The FY 2014 President's Budget estimate is $237.522 million, an increase of $3.208 million above the FY 2012 Actual level. NINDS balances investigator initiated research and solicitations, including projects funded through the Institute's translational research and clinical trials programs. Pain continues to be a major area of emphasis, with NINDS leading coordination of NIH activities through the NIH Pain Consortium. Initiatives continuing into FY2014 focus on mechanisms of peripheral nerve damage by anti-cancer therapy, on the neurobiology of migraine, and on mechanisms, models, measurement, and management in pain research. NINDS through the Consortium also supports the NIH Pain Consortium Centers of Excellence for Pain Education, which act as hubs for the development, evaluation, and distribution of pain management curriculum resources for medical, dental, nursing and pharmacy schools to enhance and improve how health care professionals are taught about pain and its treatment.
Translational Research:
The Office of Translational Research (OTR) facilitates the preclinical discovery and development of new therapeutic interventions for neurological disorders. OTR manages NINDS translational activities that span the Institute's extramural programs and provides therapy development expertise that complements disease-specific expertise across the Institute. The largest OTR program, the Cooperative Program in Translational Research, supports teams of academic and small business investigators to develop therapies for neurological disorders. The failure rate is high in therapy development, and therefore NINDS has instituted milestone-based funding that allows OTR to stop projects in this program that are no longer making progress and to shift funds to more promising opportunities. In its first decade, eight projects from this program have filed Investigative New Drug applications with the FDA; three are now in NINDS clinical trials and others are moving forward in the private sector. OTR also directs the longstanding Anticonvulsant Screening Program, which has contributed to the development of eight drugs now on the market for epilepsy. OTR is leading the NIH Blueprint for Neuroscience Grand Challenge on Neurotherapeutics, which is developing novel drugs that could transform the treatment of nervous system diseases. OTR also leads the NINDS SBIR and STTR programs, working with scientific experts throughout the extramural program as with other translational activities. NINDS continues an active working group with the FDA Center for Biologics Research to discuss shared interests on advancing cell, gene therapy, and other biologic therapies for neurological disorders. NINDS is also taking an active role in NIH-FDA regulatory science initiatives.
Budget Policy: The FY 2014 President's Budget estimate is $85.538 million, an increase of $1.155 million above the FY 2012 Actual level. This includes programs led by the Office of Translational Research, but does not include all NINDS translation research activities, which are also supported through budgets of other program areas as appropriate to the disease of focus. A 2014 NINDS initiative will focus on developing strategies to deliver therapeutic agents through the blood brain barrier. Overcoming this major obstacle to potentially therapeutic agents has the potential to catalyze therapy development for many diseases in the public and private sector. The Cooperative Program in Translational Research continues, with solicitations reissued in 2013 for academia and small businesses, including large projects designed to reach IND, as well as smaller exploratory or developmental projects. Based on an intensive review by a panel of experts from academia, industry, and the public, NINDS is reinvigorating the Anticonvulsant Screening Program (ASP), including new leadership, to improve the program's effectiveness and focus on current patient needs and scientific opportunities. The NIH Neuroscience Blueprint Grand Challenge in Neurotherapeutics is continuing, with several preclinical therapy development projects underway. NINDS also continues to support SBIR/STTR grants through general and targeted solicitations related to the Institute's mission.
Intramural Research:
The NINDS Intramural Research Program conducts basic, translational, and clinical research on the NIH campus in Bethesda, Maryland. The Intramural Program benefits from close interactions across the NIH, which is home to one of the largest communities of neuroscientists in the world, including over 200 laboratories in 11 different institutes. Among the unique resources of the NIH campus, the Mark O. Hatfield Clinical Center is a hospital totally dedicated to clinical research and the NIH Porter Neuroscience Research Center integrates neuroscience across institutes and disciplinary boundaries. NINDS Intramural research on the normal nervous system covers a broad range of neuroscience, including ion channel structure and function, reconstruction of the intricate details of nervous system anatomy, neuronal development, and integrative neuroscience. Intramural laboratories conduct research and therapy development for many neurological disorders, including neurogenetic diseases, infectious diseases such as HIV, movement disorders, multiple sclerosis, epilepsy, stroke, and brain tumors. The NINDS Intramural program continues to be a leader in development of novel magnetic resonance imaging (MRI) strategies for detecting normal and abnormal function of the brain. Intramural researchers are also engaged in the Center for Neuroscience and Regenerative Medicine, which is a collaborative program with the Department of Defense, including the Walter Reed National Military Medical Center and the Uniformed Services University, which brings together clinicians and scientists across disciplines to catalyze innovative approaches to traumatic brain injury research.
Budget Policy: The FY 2014 President's Budget estimate is $157.633 million, the same as the FY 2012 Actual level. In FY2014, NINDS will activate the second phase of the Porter Neuroscience Center. This facility houses the Neuroscience programs from eight NIH Institutes. With this new facility, NINDS will build on its existing strengths in structural biology, cell biology, synapses, brain circuits, and neuro-genetics, and strengthen programs in neurodevelopment and tissue repair. In addition, NINDS will continue to build its clinical programs in Stroke, Neurosurgery, Neuromuscular disorders, neuro-immunology, and Movement Disorders. The development of a Clinical Trials group will enhance intramural first-in human clinical trials across the intramural program.
Research Management and Support (RMS):
NINDS RMS activities provide administrative, budgetary, logistical, and scientific support in the review, award, and monitoring of research grants, training awards, and research and development contracts. RMS functions also encompass strategic planning, coordination, and evaluation of the Institute's programs, regulatory compliance, international coordination, and liaison with other Federal agencies, Congress, and the public.
Budget Policy: The FY 2014 President's Budget estimate is $58.157 million, the same as the FY 2012 Actual level. The apparent increase in estimated FY 2014 FTE compared to the FY 2012 actual FTE usage level is due to the effect of transferring positions previously funded from a centralized support operation (Division of Extramural Activities Support) to individual ICs as of year-end 2012. As a result of the DEAS transfer, estimated salaries and benefits for FY 2014 are proportionately higher than those identified for FY 2012 and previous years.
Budget Authority by Object Class
(Dollars in Thousands)
| F Y 2012 Actual |
F Y 2014 PB |
Increase or Decrease |
|
|---|---|---|---|
| Total compensable workyears: | |||
| Full-time employment | 512 | 546 | 34 |
| Full-time equivalent of overtime and holiday hours | 0 | 0 | 0 |
| Average ES salary (in whole dollars) | $179,700 | $179,700 | $0 |
| Average GM/GS grade | 13.4 | 12.8 | (0.6) |
| Average GM/GS salary (in whole dollars) | $97,936 | $92,341 | ($5,595) |
| Average salary, grade established by act of July 1, 1944 (42 U.S.C. 207) (in whole dollars) |
$99,660 | $99,660 | $0 |
| Average salary of ungraded positions (in whole dollars) | $106,974 | $106,974 | $0 |
| OBJECT CLASSES | F Y 2012 Actual |
F Y 2014 PB |
Increase or Decrease |
|---|---|---|---|
| Personnel Compensation: | |||
| 11.1 Full-time permanent | $28,869 | $32,634 | $3,765 |
| 11.3 Other than full-time permanent | 23,595 | 24,627 | 1,032 |
| 11.5 Other personnel compensation | 1,339 | 1,498 | 159 |
| 11.7 Military personnel | 540 | 559 | 19 |
| 11.8 Special personnel services payments | 7,239 | 7,360 | 121 |
| Total, Personnel Compensation | $61,582 | $66,678 | $5,096 |
| 12.0 Personnel benefits | $16,094.70 | $17,485 | $1,390 |
| 12.2 Military personnel benefits | 375 | 385 | 10 |
| 13.0 Benefits for former personnel | 0 | 0 | 0 |
| Subtotal, Pay Costs | $78,052 | $84,548 | $6,496 |
| 21.0 Travel and transportation of persons | $3,556.52 | $3,556 | ($1) |
| 22.0 Transportation of things | 245 | 244 | (1) |
| 23.1 Rental payments to GSA | 1 | 1 | 0 |
| 23.2 Rental payments to others | 80 | 79 | (1) |
| 23.3 Communications, utilities and miscellaneous charges | 886 | 886 | 0 |
| 24.0 Printing and reproduction | 36 | 36 | 0 |
| 25.1 Consulting services | 4,949 | 4,949 | 0 |
| 25.2 Other services | 22,222 | 15,080 | (7,142) |
| 25.3 Purchase of goods and services from government accounts | 131,534 | 133,278 | 1,744 |
| 25.4 Operation and maintenance of facilities | 1,411 | 1,411 | 0 |
| 25.5 Research and development contracts | 26,373 | 25,276 | (1,097) |
| 25.6 Medical care | 571 | 571 | (0) |
| 25.7 Operation and maintenance of equipment | 11,237 | 11,237 | (0) |
| 25.8 Subsistence and support of persons | 0 | 0 | 0 |
| 25.0 Subtotal, Other Contractual Services | $198,298 | $191,802 | ($6,496) |
| 26.0 Supplies and materials | $9,311.54 | $9,312 | $0 |
| 31.0 Equipment | 10,086 | 10,086 | 0 |
| 32.0 Land and structures | 0 | 0 | (0) |
| 33.0 Investments and loans | 0 | 0 | 0 |
| 41.0 Grants, subsidies and contributions | 1,322,793 | 1,342,068 | 19,275 |
| 42.0 Insurance claims and indemnities | 0 | 0 | 0 |
| 43.0 Interest and dividends | 1 | 1 | 0 |
| 44.0 Refunds | 0 | 0 | 0 |
| Subtotal, Non-Pay Costs | $1,545,291 | $1,558,071 | $12,780 |
| Total Budget Authority by Object Class | $1,623,344 | $1,642,619 | $19,275 |
Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
Salaries and Expenses
(Dollars in Thousands)
| OBJECT CLASSES | F Y 2012 Actual |
F Y 2014 PB |
Increase or Decrease |
|---|---|---|---|
| Personnel Compensation: | |||
| Full-time permanent (11.1) | $28,869 | $32,634 | $3,765 |
| Other than full-time permanent (11.3) | 23,595 | 24,627 | 1,032 |
| Other personnel compensation (11.5) | 1,339 | 1,498 | 159 |
| Military personnel (11.7) | 540 | 559 | 19 |
| Special personnel services payments (11.8) | 7,239 | 7,360 | 121 |
| Total Personnel Compensation (11.9) | $61,582 | $66,678 | $5,096 |
| Civilian personnel benefits (12.1) | $16,095 | $17,485 | $1,390 |
| Military personnel benefits (12.2) | 375 | 385 | 10 |
| Benefits to former personnel (13.0) | 0 | 0 | 0 |
| Subtotal, Pay Costs | $78,052 | $84,548 | $6,496 |
| Travel (21.0) | $3,557 | $3,556 | ($1) |
| Transportation of things (22.0) | 245 | 244 | (1) |
| Rental payments to others (23.2) | 80 | 79 | (1) |
| Communications, utilities and miscellaneous charges (23.3) | 886 | 886 | 0 |
| Printing and reproduction (24.0) | 36 | 36 | 0 |
| Other Contractual Services: | |||
| Advisory and assistance services (25.1) | 4,949 | 4,949 | 0 |
| Other services (25.2) | 22,222 | 15,080 | (7,142) |
| Purchases from government accounts (25.3) | 91,581 | 86,933 | (4,648) |
| Operation and maintenance of facilities (25.4) | 1,411 | 1,411 | 0 |
| Operation and maintenance of equipment (25.7) | 11,237 | 11,237 | 0 |
| Subsistence and support of persons (25.8) | 0 | 0 | 0 |
| Subtotal Other Contractual Services | $131,400 | $119,610 | ($11,790) |
| Supplies and materials (26.0) | $9,257 | $9,257 | $0 |
| Subtotal, Non-Pay Costs | $145,461 | $133,668 | ($11,793) |
| Total, Administrative Costs | $223,513 | $218,216 | ($5,297) |
Details of Full-Time Equivalent Employment (FTE's)
| OFFICE/DIVISION | F Y 2012 Actual |
F Y 2013 CR |
F Y 2014 PB |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Civilian | Military | Total | Civilian | Military | Total | Civilian | Military | Total | |
| Office of the Director | |||||||||
| Direct: | 48 | - | 48 | 48 | - | 48 | 48 | - | 48 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 48 | - | 48 | 48 | - | 48 | 48 | - | 48 |
| Division of Extramural Research | |||||||||
| Direct: | 92 | - | 92 | 120 | - | 120 | 120 | - | 120 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 92 | - | 92 | 120 | - | 120 | 120 | - | 120 |
| Division of Intramural Research | |||||||||
| Direct: | 338 | 5 | 343 | 338 | 5 | 343 | 338 | 5 | 343 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 338 | 5 | 343 | 338 | 5 | 343 | 338 | 5 | 343 |
| Office of Translational Research | |||||||||
| Direct: | 11 | - | 11 | 14 | - | 14 | 14 | - | 14 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 11 | - | 11 | 14 | - | 14 | 14 | - | 14 |
| Office of Clinical Research | |||||||||
| Direct: | 16 | - | 16 | 18 | - | 18 | 18 | - | 18 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 16 | - | 16 | 18 | - | 18 | 18 | - | 18 |
| Office of Special Programs in Diversity | |||||||||
| Direct: | 2 | - | 2 | 3 | - | 3 | 3 | - | 3 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 2 | - | 2 | 3 | - | 3 | 3 | - | 3 |
| Total | 507 | 5 | 512 | 541 | 5 | 546 | 541 | 5 | 546 |
Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
FTEs supported by funds from Cooperative Research and Development Agreements.
| FISCAL YEAR | Average GS Grade |
|---|---|
| 2010 | 11.8 |
| 2011 | 11.8 |
| 2012 | 13.4 |
| 2013 | 12.8 |
| 2014 | 12.8 |
Detail of Positions
(Dollars in Thousands)
| GRADE | F Y 2012 Actual |
F Y 2013 CR |
F Y 2013 PB |
|---|---|---|---|
| Total, ES Positions | 1 | 1 | 1 |
| Total, ES Salary | 179,700 | 179,700 | 179,700 |
| GM/GS-15 | 27 | 27 | 27 |
| GM/GS-14 | 50 | 50 | 50 |
| GM/GS-13 | 81 | 82 | 82 |
| GS-12 | 59 | 64 | 64 |
| GS-11 | 32 | 32 | 32 |
| GS-10 | 6 | 6 | 6 |
| GS-9 | 24 | 27 | 27 |
| GS-8 | 18 | 25 | 25 |
| GS-7 | 1 | 15 | 15 |
| GS-6 | 1 | 3 | 3 |
| GS-5 | 0 | 2 | 2 |
| GS-4 | 1 | 1 | 1 |
| GS-3 | 1 | 1 | 1 |
| GS-2 | 0 | 0 | 0 |
| GS-1 | 0 | 0 | 0 |
| Subtotal | 301 | 335 | 335 |
| Grades established by Act of July 1, 1944 (42 U.S.C. 207): | |||
| Assistant Surgeon General | 0 | 0 | 0 |
| Director Grade | 2 | 2 | 2 |
| Senior Grade | 1 | 1 | 1 |
| Full Grade | 1 | 1 | 1 |
| Senior Assistant Grade | 0 | 0 | 0 |
| Assistant Grade | 0 | 0 | 0 |
| Subtotal | 4 | 4 | 4 |
| Ungraded | 220 | 220 | 220 |
| Total permanent positions | 316 | 350 | 350 |
| Total positions, end of year | 526 | 550 | 550 |
| Total full-time equivalent (FTE) employment, end of year | 512 | 546 | 546 |
| Average ES salary | 179,700 | 179,700 | 179,700 |
| Average GM/GS grade | 13.4 | 12.8 | 12.8 |
| Average GM/GS salary | 97,936 | 92,341 | 92,341 |
Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
