
Audrey Winkelsas, DPhil
MD Candidate
University of Michigan
Serving my community through translational research
Research is essentially solving puzzles, and since I’ve always enjoyed problem-solving, my motivation to pursue science as a career came to me early on. Having a neuromuscular disease requires problem-solving as well as creativity and perseverance to be second nature. I was born with spinal muscular atrophy (SMA) and am close with the SMA community. As a high school student, I saw science as an opportunity for me to serve my community and make an impact. While an undergraduate at the University of Miami, I became involved in research at The Scripps Research Institute in the laboratory of Dr. Matthew Disney. Here, I fell in love with RNA and realized this was the direction I wanted to take with my research career. This led me to apply to the NIH Oxford-Cambridge Scholars Program for my DPhil where I was able to continue my work on RNA and developing RNA therapeutics for neuromuscular diseases.
In the NIH Oxford-Cambridge Scholars Program, graduate students divide their time between two laboratories: one at the NIH and one at the University of Oxford or the University of Cambridge. As a doctoral student, I worked in the laboratory of Dr. Kenneth Fischbeck at the National Institute of Neurological Disorders and Stroke (NINDS) and the laboratory of Dr. Matthew Wood at the University of Oxford. While these labs shared a similar research goal and were complementary to one another, the research approach and the resources available differed at each institution. Being a member of two labs exposed me to two distinct research cultures and a diversity of perspectives, which helped me grow as a researcher. Interacting with two networks of scientists helped strengthen my skills in teamwork and collaboration. I was able to establish relationships with postdoctoral fellows and other graduate students at these institutions which became an important support network during my training.
I was also fortunate to have two research mentors who were supportive of my personal and professional goals. My mentors were intentional in providing me professional development opportunities to mature as a scientist, from learning to peer-review manuscripts to presenting at conferences and seminars. In particular, Dr. Fischbeck helped me refine my communication skills. Science communication is so important because ultimately, when we make discoveries in the lab, we must be able to communicate them with our community to advance the field. Under his mentorship, I learned to present my work in a simple and concise manner, allowing the science to speak for itself and also making it more digestible for different audiences. In addition to Drs. Fischbeck and Wood, I was also informally mentored by Dr. Vivian Cheung, a visiting scientist at NINDS, who offered me a broader perspective about career development and the logistics of reaching my career goals. In the NIH Oxford-Cambridge Scholars Program, there are opportunities for many different levels of mentorship, and the culmination of these interactions helped shape me into the scientist I am today.
My graduate experience at NINDS also led me to pursue medical school after finishing my DPhil. Currently, I am an MD candidate at the University of Michigan. My time as a medical student is supported by the NIH through the Medical Scientist Training Program (MSTP) and is a continuation of the training I started in the NINDS Intramural Research Program. My ultimate career goal has not changed: I still want to understand the pathogenesis of different neuromuscular diseases and develop therapeutics for patients with neuromuscular diseases. I realized in graduate school, though, that I want to be able to bring the insights made in the lab back to the patient community as therapeutics through clinical trials.
Growing up, I was always interested in medicine, but I didn’t think it was possible or practical for me to pursue because of my disability. I didn’t see clinicians who had the same level of physical ability as me, so I decided to strictly focus on research training. While at NINDS, I experienced the clinical/translational research cycle: 1) see a patient in the clinic, 2) take a sample from the patient back to the laboratory to identify the genetic cause of their disease, 3) develop a potential therapeutic strategy for this genetic mutation in the laboratory, and 4) complete the cycle by bringing that potential therapeutic back to the patient via a clinical trial. I saw several different examples of this while in the Neurogenetics Branch at NINDS, and this made my interest in medicine resurface. I started to investigate this path more, and actively sought out and spoke with clinicians with disabilities. They showed me that being a physician-scientist was an achievable goal.
Throughout my research journey, the lack of representation is not the only obstacle I’ve faced. In general, there were a lot of logistical hurdles in the lab. For example, I am not physically able to perform benchwork and require lab assistants. I also have a service dog. When I first came to the NIH, service animals were new territory, so it took some time to figure out how to accommodate my service dog in the lab. An important skill I developed as a researcher with a disability is to advocate for myself. It’s a skill I still need to work on strengthening because it is so essential. For those considering the path of graduate school, know that the field of science needs diversity of perspective and people from all sorts of different backgrounds. Even if you don’t see someone who looks like you in an established research or clinical position, if you are passionate about science and/or medicine, I encourage you to go for it. Your background, interests, and perspective can give you a unique way of thinking about the science, and we need that breadth to successfully problem-solve together. As a future physician-scientist, I hope to not only contribute to innovation in therapeutics development, but I would also like to use my background and experiences to prioritize the needs of the patient community in clinical research in the form of more sensitive outcome measures and standards of care to help enhance their quality of life.
Current Work
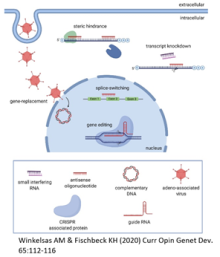
Nucleic acid therapeutics allow sequence-based targeting of genes. They can be used to increase the expression and function of disease genes or to decrease the expression of toxic gene products. One disease amenable to this treatment approach is spinal muscular atrophy (SMA). SMA is a neuromuscular disorder caused by loss of function mutations in the survival motor neuron 1 gene (SMN1). All patients have at least one copy of a paralog, SMN2, but a C-to-T transition in this gene results in exon 7 skipping in most transcripts. As a result, only 10 to 20 percent of SMN2 transcripts encode the fully functional SMN protein. Increasing the total pool of SMN2 transcripts and increasing the translational efficiency of these transcripts are strategies to increase SMN levels. The 5’ untranslated region (5’UTR) of messenger RNAs (mRNAs) regulates the transport, stability, and translational efficiency of transcripts. We identified antisense oligonucleotides targeting the 5’UTR of SMN2 that increase SMN2 mRNA and protein levels in patient cells by stabilizing SMN2 mRNA.
In future research, I plan to continue investigating the use of nucleic acid therapeutics for the treatment of rare diseases.
