ion channels; intrinsic excitability; neurophysiology; temporal coding; KCNMA1 channelopathy, an ultra-rare disorder characterized by neuromuscular dysfunction and dyskinesia, epilepsy, and neurodevelopmental delays
Andrea Meredith, Ph.D. joined NINDS in November 2024, as the Director of the Division of Extramural Activities (DEA). In this role, Dr. Meredith oversees the coordination of NINDS extramural program. She leads a team of eight branch chiefs, each playing a vital role in advancing the goals of the extramural program.
The Programmatic Operations Team oversees the receipt and referral of NINDS applications, development of new funding opportunities (NOFOs), administration of NINDS programs such as the R35 and RM1, and extramural process and programmatic analysis. The NINDS Council Team runs three NINDS Advisory Council meetings annually, where funding award decisions are finalized, and assists in the management of other Federal Advisory Committee Act (FACA)-related committee meetings. Funding awards for grants, cooperative agreements, contracts, and clinical trials are administered by the Grants Management Branch, which ensures award compliance with federal laws, regulations, and policies. The Office of Training and Workforce Development builds and supports the national neuroscience skill base and workforce infrastructure through a variety of specialized NINDS training programs. The Office of Research Quality and the Data Modeling and Analytics Team each house programs that underpin rigorous, high-quality scientific research, transparent reporting, data management, and AI initiatives.
Prior to joining NINDS, Dr. Meredith was a Professor of Physiology at the University of Maryland School of Medicine in Baltimore for more than two decades. She headed the KCNMA1 Translational Research Laboratory, an NIH-funded research lab focused on understanding a new neurogenetic disorder. Her research established the genetic curation, clinical manifestations, mechanistic underpinnings, and gene variant-delineated treatment for KCNMA1 Channelopathy, an ultra-rare seizure and dyskinesia disorder resulting from aberrant electrical signaling in the brain and muscles. She has authored over 60 peer-reviewed publications and co-founded the KCNMA1 International Advocacy Foundation (KCIAF). Dr. Meredith has also worked with clinical, NIH, industry, and patient partners to raise awareness of channelopathy disorders. She served on numerous NIH scientific review panels, scientific advisory boards, and patient advocacy committees and participated in many types of outreach activities that educate the public about brain health and neurological disorders
Dr. Meredith studies how ion channels regulate information coding in dynamic systems by combining the genetic manipulation of ion channels with electrophysiology and in vivo brain and muscle physiology. Dr. Meredith's lab has focused on the BK K+ channel, identifying biophysical gating properties critical to patterning neuronal activity in neuromuscular function and behavior.
Research Interests
BK Channels (KCNMA1)
Dr. Meredith studies the biophysical basis for neurological coding in dynamic physiological and behavioral systems, combining genetic manipulation of ion channels with electrophysiology

and in vivo brain and muscle recordings. Her lab’s studies focus on BK (Big K+) large conductance voltage and Ca2+-activated K+ channel. BK channels are biophysically well-characterized and possess several exceptional properties that contribute to their potent and state-dependent influence on membrane excitability. These include their large conductance, allosteric voltage- and Ca2+-dependent gating, cell-specific tuning through multiple molecular mechanisms, and wide expression across tissue types with diverse functions. The BK channel pore-forming alpha subunit is encoded by a single gene (KCNMA1 in humans, Slo in mice, and Slowpoke flies). Like other Kv family members, BK channels are comprised of a tetramer of alpha subunits, modulatory beta (β1-4) and gamma subunits (ɣ1-4 or LRRC26, 52, 55, and 38), and are closely localized with intracellular Ca2+ sources such as voltage-gated Ca2+ channels (VGCCs and NMDARs) and Ryanodine receptor channels (RyRs).
Dr. Meredith created variety of transgenic mouse lines (Kcnma1N999S, Kcnma1D434G, Kcnma1H444Q, Kcnma1–/–, Tg-BKR207Q, and Kcnma1flox-tdTomato) to study BK channel physiology and pathophysiology, in systems ranging from circadian rhythm to cardiac rhythm, and motor function, urodynamics, reproductive function, neurovascular coupling, hearing, and human (KCNMA1 Channelopathy) and animal (Ryegrass Staggers) neurological diseases. Prior to joining NINDS, Dr. Meredith’s lab was supported by the National Institute of Neurological Disorders and Stroke; the BRAIN Initiative®; the National Heart, Lung, and Blood Institute; the National Institute of Diabetes and Digestive and Kidney Disorders; the National Institute of General Medical Sciences; the National Institute of Mental Health; and the American Heart Association.

KCNMA1-Linked Channelopathy

BK channel dysfunction is associated with a debilitating neurological syndrome called KCNMA1 channelopathy. This ultra-rare disorder results from mutations in KCNMA1, and both gain-of-function (GOF) and loss-of-function (LOF) variants are associated with seizures, dyskinesia, neurodevelopmental delay, intellectual disability, and brain and structural malformations. We study novel patient-associated
KCNMA1 variants within the BK channel structure and their functional classifications by electrophysiology to validate genotype-phenotype associations, disease models, and treatment possibilities. More than half of affected individuals present with a rare negative episodic motor disorder, paroxysmal non-kinesigenic dyskinesia (PNKD3), associated with three GOF variants, and reported a therapeutic approach for PNKD3. Introduction of two of these GOF KCNMA1 variants in mouse disease models recapitulated seizure susceptibility, paroxysmal dyskinesia, and the therapeutic response, providing initial insights into the neurological basis. Our current priorities focus on the curation of KCNMA1 variants, functional characterization in BK channels, and understanding the channelopathy disease mechanisms.
Patients and families can find out more about KCNMA1 channelopathy in the Patient Resources section below.
Circadian Rhythm
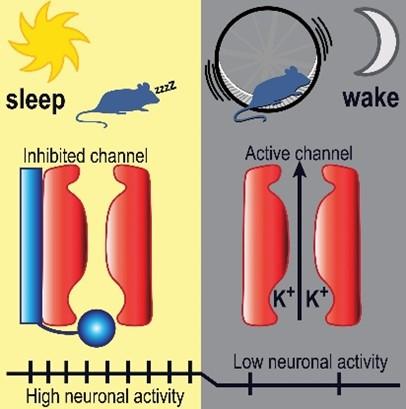
The neural coding of circadian time in the brain’s clock is a highly tractable system for investigating the molecular and biophysical mechanisms that drive state-dependent changes in excitability. Daily physiological and behavioral rhythms (~24 hrs) are a universal trait of animals, vital for adaptation, fitness, and survival. In mammals, lesion and transplantation studies have localized the principal circadian pacemaker to the suprachiasmatic nucleus (SCN) of the hypothalamus, identifying a discrete neural substrate for a complex behavior. We identified a novel role for the BK channel in the daily patterning of SCN neural activity. Slo/Kcnma1–/– mice have degraded circadian behavioral and physiological rhythms, and their SCN neurons exhibit aberrant SCN action potential and circuit rhythmicity.
We discovered that daily modulation of BK channel properties is necessary for SCN circuit rhythmicity. We identified circadian modulation of the expression, alternative splicing, β subunit regulation, and Ca2+ channel dependence producing diurnal changes in BK channel activity. These mechanisms underlie time-of-day specific roles for BK channels that shape the neural representation of circadian time in the SCN. Aberrant elevation of the BK current during the day in the forces the circuit into a ‘down-state,’ decreasing rhythmicity and altering clock-controlled behavioral. These studies reveal that circadian modulation of ionic currents is critical for circadian rhythm and offer a novel entry point for the treatment of sleep and circadian disorders.
Rhythmic Excitability
Building on the role for BK channels in central circadian time encoding, we have also investigated the contribution of BK channels to other types of rhythmic excitability. We identified intrinsic day-night differences in bladder urodynamics in a behaviorally-integrated rodent model. In urinary bladder smooth muscle (UBSM), BK channels are critical for excitability, and isolated UBSM strips exhibit a diurnal difference in contractile activity that is phased with BK channel expression.
BK channels also regular other rhythmic systems on faster timescales, such as sinoatrial node. In spontaneously firing sinoatrial node cells, BK channels regulate pacemaker frequency and consequently influence heart rate. Inhibiting BK channels causes a counter-intuitive decrease in heart rate (bradycardia), which is absent in mice lacking BK channels.
Additional studies have investigated the rhythmicity of voltage-gated Ca2+ currents in SCN and the temporal and spatial dynamics of VGCCs in circuit rhythms using optical imaging of quantitative FRET biosensors.
Patient Resources
KCNMA1 Channelopathy International Advocacy Foundation
NIH The Undiagnosed Diseases Program
Meredith Lab website (2006-2024), University of Maryland School of Medicine
Publications
KCNMA1 Channelopathy
Dinsdale RL, Middendorf TR, Disilvestre D, Adams D, Gahl W, Macnamara EF, Wolfe L, Toro C, Tifft CJ, Meredith AL (2025) BK channel activity in skin fibroblasts from patients with neurological disorder.
Channels (Austin), 19:2542811. PubMed ID: 40785052
Dinsdale RL, Meredith AL (2024) Evaluation of four KCNMA1 channelopathy variants on BK channel current under CaV1.2 activation. Channels (Austin), 18:2396346. PubMed ID: 39217512
Meredith AL (2024) BK Channelopathies and KCNMA1-Linked Disease Models. Annu Rev Physiol, 86:277-300. PubMed ID: 37906945
Moldenhauer HJ, Tammen K, Meredith AL (2024)
Structural mapping of patient-associated KCNMA1 gene variants.
Biophys J, 123:1984-2000. PubMed ID: 38042986
Park SM, Roache CE, Iffland PH, Moldenhauer HJ, Matychak KK, Plante AE, Lieberman AG, Crino PB, Meredith A (2022)
BK channel properties correlate with neurobehavioral severity in three KCNMA1-linked channelopathy mouse models.
Elife, 11 PubMed ID: 35819138
Moldenhauer HJ, Dinsdale RL, Alvarez S, Fernández-Jaén A, Meredith AL (2022) Effect of an autism-associated KCNMB2 variant, G124R, on BK channel properties.
Curr Res Physiol, 5:404-413. PubMed ID: 36203817
Keros S, Heim J, Hakami W, Zohar-Dayan E, Ben-Zeev B, Grinspan Z, Kruer MC, Meredith AL (2022) Lisdexamfetamine Therapy in Paroxysmal Non-kinesigenic Dyskinesia Associated with the KCNMA1-N999S Variant. Mov Disord Clin Pract, 9:229-235. PubMed ID: 35141357
Miller JP, Moldenhauer HJ, Keros S, Meredith AL (2021) An emerging spectrum of variants and clinical features in KCNMA1-linked channelopathy. Channels (Austin), 15:447-464. PubMed ID: 34224328
Buckley C, Williams J, Munteanu T, King M, Park SM, Meredith AL, Lynch T (2020) Status Dystonicus, Oculogyric Crisis and Paroxysmal Dyskinesia in a 25 Year-Old Woman with a Novel KCNMA1 Variant, K457E. Tremor Other Hyperkinet Mov (N Y), 10:49. PubMed ID: 33178487
Heim J, Vemuri A, Lewis S, Guida B, Troester M, Keros S, Meredith A, Kruer MC (2020) Cataplexy in Patients Harboring the KCNMA1 p.N999S Mutation. Mov Disord Clin Pract, 7:861-862. PubMed ID: 33043086
Bailey CS, Moldenhauer HJ, Park SM, Keros S, Meredith AL (2019) KCNMA1-linked channelopathy. J Gen Physiol, 151:1173-1189. PubMed ID: 31427379
Moldenhauer, HJ, Park, SM, and Meredith, AL (2020). Characterization of new human KCNMA1 Loss-of-Function Mutations. Biophysical Journal 114A.
Moldenhauer HJ, Matychak KK, Meredith AL (2020) Comparative gain-of-function effects of the KCNMA1-N999S mutation on human BK channel properties.
J Neurophysiol, 123:560-570. PubMed ID: 31851553
Plante AE, Lai MH, Lu J, Meredith AL (2019) Effects of Single Nucleotide Polymorphisms in Human KCNMA1 on BK Current Properties. Front Mol Neurosci, 12:285. PubMed ID: 31849601
Montgomery JR, Meredith AL (2012) Genetic activation of BK currents in vivo generates bidirectional effects on neuronal excitability. Proc Natl Acad Sci U S A, 109:18997-9002. PubMed ID: 23112153
Imlach WL, Finch SC, Dunlop J, Meredith AL, Aldrich RW, Dalziel JE (2008) The molecular mechanism of "ryegrass staggers," a neurological disorder of K+ channels.
J Pharmacol Exp Ther, 327:657-64. PubMed ID: 18801945
Circadian Rhythm
Dinsdale RL, Roache CE, Meredith AL (2023) Disease-associated KCNMA1 variants decrease circadian clock robustness in channelopathy mouse models.
J Gen Physiol, 155 PubMed ID: 37728576
McNally BA, Plante AE, Meredith AL (2021) Contributions of CaV1.3 Channels to Ca2+ Current and Ca2+-Activated BK Current in the Suprachiasmatic Nucleus.
Front Physiol, 12:737291. PubMed ID: 34650447
Plante AE, Rao VP, Rizzo MA, Meredith AL (2021) Comparative Ca2+ channel contributions to intracellular Ca2+ levels in the circadian clock. Biophys Rep (N Y), 1 PubMed ID: 35330949
Plante AE, Whitt JP, Meredith AL (2021) BK channel activation by L-type Ca2+ channels CaV1.2 and CaV1.3 during the subthreshold phase of an action potential.
J Neurophysiol, 126:427-439. PubMed ID: 34191630
Harvey JRM, Plante AE, Meredith AL (2020) Ion Channels Controlling Circadian Rhythms in Suprachiasmatic Nucleus Excitability. Physiol Rev, 100:1415-1454. PubMed ID: 32163720
McNally BA, Plante AE, Meredith AL (2020) Diurnal properties of voltage-gated Ca2+ currents in suprachiasmatic nucleus and roles in action potential firing.
J Physiol, 598:1775-1790. PubMed ID: 31177540
Whitt JP, McNally BA, Meredith AL (2018) Differential contribution of Ca2+ sources to day and night BK current activation in the circadian clock.
J Gen Physiol, 150:259-275. PubMed ID: 29237755
- ‘Up all night: BK channels’ circadian dance with different calcium sources. JGP 150(2):175.
Whitt JP, Montgomery JR, Meredith AL (2016) BK channel inactivation gates daytime excitability in the circadian clock. Nat Commun, 7:10837. PubMed ID: 26940770
White RS, Zemen BG, Khan Z, Montgomery JR, Herrera GM, Meredith AL (2014) Evaluation of mouse urinary bladder smooth muscle for diurnal differences in contractile properties. Front Pharmacol, 5:293. PubMed ID: 25620932
Shelley C, Whitt JP, Montgomery JR, Meredith AL (2013) Phosphorylation of a constitutive serine inhibits BK channel variants containing the alternate exon "SRKR".
J Gen Physiol, 142:585-98. PubMed ID: 24277602
Hermanstyne TO, Subedi K, Le WW, Hoffman GE, Meredith AL, Mong JA, Misonou H (2013) Kv2.2: a novel molecular target to study the role of basal forebrain GABAergic neurons in the sleep-wake cycle. Sleep, 36:1839-48. PubMed ID: 24293758
Montgomery JR, Whitt JP, Wright BN, Lai MH, Meredith AL (2013) Mis-expression of the BK K(+) channel disrupts suprachiasmatic nucleus circuit rhythmicity and alters clock-controlled behavior. Am J Physiol Cell Physiol, 304:C299-311. PubMed ID: 23174562
White RS, Zemen BG, Khan Z, Montgomery JR, Herrera GM, Meredith AL (2014) Evaluation of mouse urinary bladder smooth muscle for diurnal differences in contractile properties. Front Pharmacol, 5:293. PubMed ID: 25620932
Herrera GM, Meredith AL (2010) Diurnal variation in urodynamics of rat. PLoS One, 5:e12298. PubMed ID: 20808873
Kent J, Meredith AL (2008) BK channels regulate spontaneous action potential rhythmicity in the suprachiasmatic nucleus.
PLoS One, 3:e3884. PubMed ID: 19060951
Meredith AL, Wiler SW, Miller BH, Takahashi JS, Fodor AA, Ruby NF, Aldrich RW (2006) BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nat Neurosci, 9:1041-9. PubMed ID: 16845385
Neurovascular, Cardiovascular and Smooth Muscle Excitability
Lai MH, Wu Y, Gao Z, Anderson ME, Dalziel JE, Meredith AL (2014) BK channels regulate sinoatrial node firing rate and cardiac pacing in vivo.
Am J Physiol Heart Circ Physiol, 307:H1327-38. PubMed ID: 25172903
Zemen BG, Lai MH, Whitt JP, Khan Z, Zhao G, Meredith AL (2015) Generation of Kcnma1fl-tdTomato, a conditional deletion of the BK channel α subunit in mouse.
Physiol Rep, 3 PubMed ID: 26537348
White RS, Zemen BG, Khan Z, Montgomery JR, Herrera GM, Meredith AL (2014) Evaluation of mouse urinary bladder smooth muscle for diurnal differences in contractile properties. Front Pharmacol, 5:293. PubMed ID: 25620932
Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT (2010) Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci U S A, 107:3811-6. PubMed ID: 20133576
Imlach WL, Finch SC, Miller JH, Meredith AL, Dalziel JE (2010) A role for BK channels in heart rate regulation in rodents. PLoS One, 5:e8698. PubMed ID: 20090847
Herrera GM, Meredith AL (2010) Diurnal variation in urodynamics of rat. PLoS One, 5:e12298. PubMed ID: 20808873
Werner ME, Knorn AM, Meredith AL, Aldrich RW, Nelson MT (2007) Frequency encoding of cholinergic- and purinergic-mediated signaling to mouse urinary bladder smooth muscle: modulation by BK channels. Am J Physiol Regul Integr Comp Physiol, 292:R616-24. PubMed ID: 16931654
Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT (2006) Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci, 9:1397-1403. PubMed ID: 17013381
Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW (2004) Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem, 279:36746-52. PubMed ID: 15184377
Additional Neuronal and Neurobehavioral Studies
Zhang J, Guan X, Li Q, Meredith AL, Pan HL, Yan J (2018) Glutamate-activated BK channel complexes formed with NMDA receptors. Proc Natl Acad Sci U S A, 115:E9006-E9014. PubMed ID: 30181277
Nelson AB, Faulstich M, Moghadam S, Onori K, Meredith A, du Lac S (2017) BK Channels Are Required for Multisensory Plasticity in the Oculomotor System.
Neuron, 93:211-220. PubMed ID: 27989457
Hayashi Y, Morinaga S, Zhang J, Satoh Y, Meredith AL, Nakata T, Wu Z, Kohsaka S, Inoue K, Nakanishi H (2016) BK channels in microglia are required for morphine-induced hyperalgesia. Nat Commun, 7:11697. PubMed ID: 27241733
Li B, Jie W, Huang L, Wei P, Li S, Luo Z, Friedman AK, Meredith AL, Han MH, Zhu XH, Gao TM (2014) Nuclear BK channels regulate gene expression via the control of nuclear calcium signaling. Nat Neurosci, 17:1055-63. PubMed ID: 24952642
Singh H, Lu R, Bopassa JC, Meredith AL, Stefani E, Toro L (2013) MitoBK(Ca) is encoded by the Kcnma1 gene, and a splicing sequence defines its mitochondrial location.
Proc Natl Acad Sci U S A, 110:10836-41. PubMed ID: 23754429
Indriati DW, Kamasawa N, Matsui K, Meredith AL, Watanabe M, Shigemoto R (2013) Quantitative localization of Cav2.1 (P/Q-type) voltage-dependent calcium channels in Purkinje cells: somatodendritic gradient and distinct somatic coclustering with calcium-activated potassium channels. J Neurosci, 33:3668-78. PubMed ID: 23426693
Maison SF, Pyott SJ, Meredith AL, Liberman MC (2013) Olivocochlear suppression of outer hair cells in vivo: evidence for combined action of BK and SK2 channels throughout the cochlea. J Neurophysiol, 109:1525-34. PubMed ID: 23282326
Pyott SJ, Meredith AL, Fodor AA, Vázquez AE, Yamoah EN, Aldrich RW (2007) Cochlear function in mice lacking the BK channel alpha, beta1, or beta4 subunits.
J Biol Chem, 282:3312-24. PubMed ID: 17135251
Misonou H, Menegola M, Buchwalder L, Park EW, Meredith A, Rhodes KJ, Aldrich RW, Trimmer JS (2006) Immunolocalization of the Ca2+-activated K+ channel Slo1 in axons and nerve terminals of mammalian brain and cultured neurons. J Comp Neurol, 496:289-302. PubMed ID: 16566008
Book Chapters
Textbook of Ion Channels (Taylor & Francis, 2022)
Genetic Models and Transgenics (Volume 1, Chapter 15)
Alternative Splicing (Volume 3, Chapter 1)