Overview
Amyotrophic lateral sclerosis (ALS) is a progressive and ultimately fatal neurodegenerative disorder that affects motor neurons responsible for initiating and controlling voluntary muscle movement and the diaphragm. Disease progression leads to worsening muscle weakness and atrophy, causing difficulty in limb movement, chewing, swallowing, speaking, and breathing. Approximately 30,000 people in the U.S. are living with ALS.1 Roughly 90% of ALS cases are sporadic, meaning they emerge with no clear family history. Despite a negative family history, people with sporadic ALS can have disease-associated in genes linked to ALS. Approximately 10% of individuals with ALS have familial ALS, typically with autosomal dominant inheritance. Dozens of ALS-linked genes have been identified, including C9orf72, FUS, TARDBP, and SOD1.2SOD1 was the first gene linked to ALS, and disease-associated variants in this gene account for 13-20% of familial ALS cases.3
In April 2023, tofersen, marketed in the U.S. as Qalsody® (Biogen), was approved by the FDA for the treatment of SOD1-linked ALS. The SOD1 gene encodes a protein called superoxide dismutase 1. SOD1 mutations linked to ALS are thought to result in a "toxic", misfolded form of the protein, which accumulates in motor neurons, disrupts cellular metabolism and other processes, and propagates between cells. Tofersen is an antisense oligonucleotide (ASO), a type of precision therapy designed to modify the expression of a specific gene. In this case, tofersen binds to normal and mutated SOD1 messenger RNA (mRNA) and prevents translation into protein, thereby reducing the overall level of SOD1 protein, including the toxic mutated form.
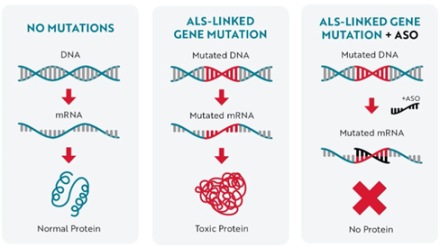
Image credit: ALS Association
Over 30 years of NIH-funded research provided pivotal discoveries crucial for the development of tofersen. In 1993, an NIH-funded study established SOD1 variants as the first known genetic cause for a subset of ALS cases.5 This discovery paved the way for studying disease mechanisms and testing potential therapies in genetically engineered mouse models of SOD1-linked ALS.6 The development of nusinersen (Spinraza®), an ASO treatment for spinal muscular atrophy (SMA), demonstrated the potential for this approach to neurodegenerative diseases. U.S. and international academic organizations, government agencies like the NIH, and private entities invested in research into a similar therapy for SOD1-related ALS, which ultimately led to the development of tofersen by Biogen and Ionis Pharmaceuticals. Meanwhile, research in the U.S. and abroad identified a separate structural protein in neurons called neurofilament light chain (NfL) as a reliable biomarker for assessing neuronal damage, disease progression, and prognosis in ALS.7 FDA approval of tofersen relied on the treatment’s ability to lower blood plasma NfL levels by 40-50% over a six-month period.8 Building on this breakthrough, the first disease prevention trial for SOD1-linked ALS is now underway to evaluate tofersen's longer term efficacy and safety. NIH continues supporting research to develop ASOs targeting ALS-associated genes and to explore other novel approaches, such as genome editing, to treat ALS.9
Timeline
Researchers link variants in the SOD1 gene to familial cases of ALS.3,10
Mice engineered with a mutated human ALS gene suggest that the resulting misfolded protein causes harm by accumulating in neurons. 6
Studies show that patients with ALS and other neurodegenerative diseases have increased levels of neurofilament light chain (NfL) protein in cerebrospinal fluid (CSF) the fluid that surrounds and protects the brain and spinal cord. These and other results suggest that NfL may be a meaningful marker of neurodegeneration.11
Researchers show that non-neuronal cells expressing mutant SOD1 protein contribute significantly to ALS pathology. This finding suggests that targeting mutant SOD1 broadly across multiple cell types may be a promising strategy for therapy development. 12
In an ALS mouse model, silencing mutated SOD1 genes protects against neurodegeneration and extends survival. 13, 14

The first ASO designed as a therapeutic strategy for SOD1-linked ALS slows disease progression in a rat model. 15
A combination of CSF biomarkers, including neurofilament proteins, shows promise for distinguishing ALS from other neurodegenerative diseases or healthy control cases. 16
A clinical study shows that neurofilament light chain (NfL) levels in blood plasma predict ALS disease progression, building on prior results in CSF and pointing to blood-derived NfL as a more accessible and less invasive biomarker.17

The first clinical trial of tofersen begins. 18
FDA approves nusinersen (Spinraza) for the treatment of SMA in children, demonstrating the potential for ASO therapies to treat neurodegenerative diseases. 19
Researchers determine that ASO therapy in ALS rodent models can extend survival and reverse neurodegeneration, even when treatment begins after disease onset.20
The phase 3 clinical trial of tofersen shows reductions in SOD1 and plasma NfL levels in treated participants but fails to show significant improvement on a functional measure of disease progression .8
FDA grants accelerated approval to tofersen (Qalsody®) for the treatment of SOD1-ALS, based on a determination that the treatment’s ability to reduce plasma NfL levels is likely to predict a clinical benefit in patients. Additional clinical trials are ongoing to evaluate tofersen’s longer term safety and efficacy, including in individuals with SOD1 variants who do not yet have symptoms.8, 21, 22

List of References
Centers for Disease Control and Prevention. National ALS Registry Dashboard. September 1, 2023. Available at https://www.cdc.gov/als/dashboard/index.html[1]. Accessed September 27, 2024. als.gov number NCT04856982)
Reviewed in: Mejzini R, Flynn LL, Pitout IL, Fletcher S, Wilton SD, Akkari PA. ALS Genetics, Mechanisms, and Therapeutics: Where Are We Now? Front Neurosci. 2019 Dec 6;13:1310. doi: 10.3389/fnins.2019.01310. PMID: 31866818; PMCID: PMC6909825. (Australia, public and private sources)
Berdyński M, Miszta P, Safranow K, Andersen PM, Morita M, Filipek S, Żekanowski C, Kuźma-Kozakiewicz M. SOD1 mutations associated with amyotrophic lateral sclerosis analysis of variant severity. Sci Rep. 2022 Jan 7;12(1):103. doi: 10.1038/s41598-021-03891-8. PMID: 34996976; PMCID: PMC8742055. (European Union and Poland, public sources)
ALS Association. Antisense Therapy for ALS. ALS Association, https://www.als.org/research/als-research-topics/genetics/antisense-therapy-for-als. Accessed July 30, 2024.
Deng HX, Hentati A, Tainer JA, Iqbal Z, Cayabyab A, Hung WY, Getzoff ED, Hu P, Herzfeldt B, Roos RP, et al. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science. 1993 Aug 20;261(5124):1047-51. doi: 10.1126/science.8351519. PMID: 8351519. (unable to locate source of support)
Show More
Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994 Jun 17;264(5166):1772-5. doi: 10.1126/science.8209258. Erratum in: Science 1995 Jul 14;269(5221):149. PMID: 8209258. (NIH/NINDS and NIA, grants NS010580, NS020471, and AG05146)
Reviewed in: Khalil M, Teunissen CE, Lehmann S, Otto M, Piehl F, Ziemssen T, Bittner S, Sormani MP, Gattringer T, Abu-Rumeileh S, Thebault S, Abdelhak A, Green A, Benkert P, Kappos L, Comabella M, Tumani H, Freedman MS, Petzold A, Blennow K, Zetterberg H, Leppert D, Kuhle J. Neurofilaments as biomarkers in neurological disorders - towards clinical application. Nat Rev Neurol. 2024 May;20(5):269-287. doi: 10.1038/s41582-024-00955-x. Epub 2024 Apr 12. PMID: 38609644. (Austria, Canada, Netherlands, Germany, Sweden, Switzerland, UK, USA, public and private sources)
Miller TM, Cudkowicz ME, Genge A, Shaw PJ, Sobue G, Chiò A, Van Damme P, Ludolph AC, Glass JD, Atassi N, Benatar M, Chary S, Chew S, Zhang H, Wu F, Nestorov I, Graham D, Sun P, Fradette L, Fanning TA, Ferguson S, Meininger V, McDermott CJ, Bali T, Boschert U, Boylan KB, Bozik ME, Browne SE, Caress JB, Chio A, Cohen J, Corti S, Cudkowicz M, Cudkowicz ME, Desai U, Eisen A, Elamin M, Ferguson T, Floeter MK, Fogh I, Forshew D, Genge A, Glass J, Gooch C, Hardiman O, Harms M, Heiman-Patterson T, Henderson R, Ingersoll E, Jenkins T, Kasarskis E, Kiernan M, Lomen-Hoerth C, Maragakis N, Meininger V, Miller R, Mora G, Mitsumoto H, Needham M, Oskarsson B, Payan C, Petri S, Pioro E, Pradat PF, Ravits J, Rosenfeld J, Rothstein J, Rutkove S, Scelsa S, Shaw P, Shefner J, Simmons Z, Smith R, Sorenson E, Swash M, Talbot K, Traynor B, Turner M, Vucic S, Weiss M, Wijesekera L, Windebank A, Wuu J, Zinman L. Trial of Antisense Oligonucleotide Tofersen for SOD1 ALS. N Engl J Med. 2022 Sep 22;387(12):1099-1110. doi: 10.1056/NEJMoa2204705. Epub 2022 Sep 21. PMID: 36129998. (Biogen Pharmaceuticals; ClinicalTrials.gov numbers, NCT02623699, NCT03070119)
Columbia University Irving Medical Center. Columbia Awarded $15 Million to Create Medicines for Ultra-Rare Forms of ALS [Internet]. New York: Columbia University Irving Medical Center; 2023 Jul 11. Available at: https://www.cuimc.columbia.edu/news/columbia-awarded-15-million-create-medicines-ultra-rare-forms-als. Accessed July 2, 2024.
Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993 Mar 4;362(6415):59-62. doi: 10.1038/362059a0. Erratum in: Nature. 1993 Jul 22;364(6435):362. doi: 10.1038/364362c0. PMID: 8446170. (NINDS funding)
Rosengren LE, Karlsson JE, Karlsson JO, Persson LI, Wikkelsø C. Patients with amyotrophic lateral sclerosis and other neurodegenerative diseases have increased levels of neurofilament protein in CSF. J Neurochem. 1996 Nov;67(5):2013-8. doi: 10.1046/j.1471-4159.1996.67052013.x. PMID: 8863508. (Sweden, private sources)
Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillée S, Rule M, McMahon AP, Doucette W, Siwek D, Ferrante RJ, Brown RH Jr, Julien JP, Goldstein LS, Cleveland DW. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 2003 Oct 3;302(5642):113-7. doi: 10.1126/science.1086071. Erratum in: Science. 2003 Oct 24;302(5645):568. PMID: 14526083. (NIH/NINDS, NICHD and NIA, grants NS31248, NS37912, NS27036, AG13846, AG12992, HD30249)
Raoul C, Abbas-Terki T, Bensadoun JC, Guillot S, Haase G, Szulc J, Henderson CE, Aebischer P. Lentiviral-mediated silencing of SOD1 through RNA interference retards disease onset and progression in a mouse model of ALS. Nat Med. 2005 Apr;11(4):423-8. doi: 10.1038/nm1207. Epub 2005 Mar 13. PMID: 15768028. (ALS Association; Swiss National Science Foundation; European Union contract LSHM-CT-2003-503330)
Ralph GS, Radcliffe PA, Day DM, Carthy JM, Leroux MA, Lee DC, Wong LF, Bilsland LG, Greensmith L, Kingsman SM, Mitrophanous KA, Mazarakis ND, Azzouz M. Silencing mutant SOD1 using RNAi protects against neurodegeneration and extends survival in an ALS model. Nat Med. 2005 Apr;11(4):429-33. doi: 10.1038/nm1205. Epub 2005 Mar 13. PMID: 15768029. (UK, Oxford Biomedica Ltd.)
Smith RA, Miller TM, Yamanaka K, Monia BP, Condon TP, Hung G, Lobsiger CS, Ward CM, McAlonis-Downes M, Wei H, Wancewicz EV, Bennett CF, Cleveland DW. Antisense oligonucleotide therapy for neurodegenerative disease. J Clin Invest. 2006 Aug;116(8):2290-6. doi: 10.1172/JCI25424. Epub 2006 Jul 27. PMID: 16878173; PMCID: PMC1518790. (NIH/NINDS, grant NS027036)
Ganesalingam J, An J, Shaw CE, Shaw G, Lacomis D, Bowser R. Combination of neurofilament heavy chain and complement C3 as CSF biomarkers for ALS. J Neurochem. 2011 May;117(3):528-37. doi: 10.1111/j.1471-4159.2011.07224.x. Epub 2011 Mar 21. PMID: 21418221; PMCID: PMC3076545. (NIH/NINDS grant NS061867; UK, private and public sources)
Lu CH, Macdonald-Wallis C, Gray E, Pearce N, Petzold A, Norgren N, Giovannoni G, Fratta P, Sidle K, Fish M, Orrell R, Howard R, Talbot K, Greensmith L, Kuhle J, Turner MR, Malaspina A. Neurofilament light chain: A prognostic biomarker in amyotrophic lateral sclerosis. Neurology. 2015 Jun 2;84(22):2247-57. doi: 10.1212/WNL.0000000000001642. Epub 2015 May 1. Erratum in: Neurology. 2015 Sep 8;85(10):921. doi: 10.1212/WNL.0000000000001986. PMID: 25934855; PMCID: PMC4456658. (UK, private and public sources)
Miller T, Cudkowicz M, Shaw PJ, Andersen PM, Atassi N, Bucelli RC, Genge A, Glass J, Ladha S, Ludolph AL, Maragakis NJ, McDermott CJ, Pestronk A, Ravits J, Salachas F, Trudell R, Van Damme P, Zinman L, Bennett CF, Lane R, Sandrock A, Runz H, Graham D, Houshyar H, McCampbell A, Nestorov I, Chang I, McNeill M, Fanning L, Fradette S, Ferguson TA. Phase 1-2 Trial of Antisense Oligonucleotide Tofersen for SOD1 ALS. N Engl J Med. 2020 Jul 9;383(2):109-119. doi: 10.1056/NEJMoa2003715. PMID: 32640130. (Biogen Pharmaceuticals; Clinicaltrials.gov number NCT02623699)
U.S. Food and Drug Administration. FDA approves first drug for spinal muscular atrophy. December 23, 2016. Available at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm534611.htm. Accessed September 17, 2024.
McCampbell A, Cole T, Wegener AJ, Tomassy GS, Setnicka A, Farley BJ, Schoch KM, Hoye ML, Shabsovich M, Sun L, Luo Y, Zhang M, Comfort N, Wang B, Amacker J, Thankamony S, Salzman DW, Cudkowicz M, Graham DL, Bennett CF, Kordasiewicz HB, Swayze EE, Miller TM. Antisense oligonucleotides extend survival and reverse decrement in muscle response in ALS models. J Clin Invest. 2018 Aug 1;128(8):3558-3567. doi: 10.1172/JCI99081. Epub 2018 Jul 16. PMID: 30010620; PMCID: PMC6063493. (NIH/NINDS, grants NS078398 and NS084970)
U.S. Food and Drug Administration. FDA approves treatment of amyotrophic lateral sclerosis associated with a mutation in the SOD1 gene. April 25, 2023. Available at https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-treatment-amyotrophic-lateral-sclerosis-associated-mutation-sod1-gene. Accessed August 12, 2024.
Benatar M, Wuu J, Andersen PM, Bucelli RC, Andrews JA, Otto M, Farahany NA, Harrington EA, Chen W, Mitchell AA, Ferguson T, Chew S, Gedney L, Oakley S, Heo J, Chary S, Fanning L, Graham D, Sun P, Liu Y, Wong J, Fradette S. Correction to: Design of a Randomized, Placebo-Controlled, Phase 3 Trial of Tofersen Initiated in Clinically Presymptomatic SOD1 Variant Carriers: the ATLAS Study. Neurotherapeutics. 2022 Sep;19(5):1686. doi: 10.1007/s13311-022-01286-9. Erratum for: Neurotherapeutics. 2022 Jul;19(4):1248-1258. doi: 10.1007/s13311-022-01237-4. PMID: 36175782; PMCID: PMC9606151. (Clinicaltrials.gov number NCT04856982)
