Overview
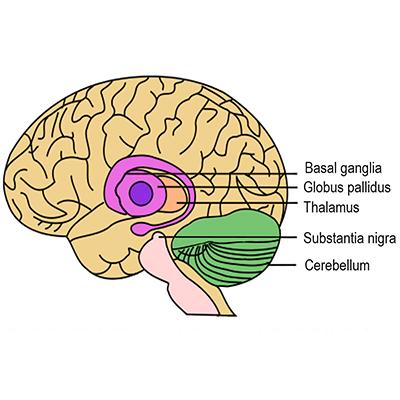
Parkinson's disease (PD) is a neurodegenerative disorder that leads to resting tremor, rigidity, slowness of movement, and postural instability. These symptoms are caused by degeneration of neurons in the substantia nigra pars compacta (SNc), one of a group of brain structures known as the basal ganglia and part of a circuit crucial for coordinating purposeful movement. This circuit relies on the chemical messenger (or neurotransmitter) dopamine, which is produced by SNc neurons. As PD progresses and these neurons are lost, reduced dopamine results in abnormal circuit activity and motor symptoms.
The molecular precursor to dopamine, L-DOPA (or levodopa), is used to treat PD. However, people in later stages of the disease experience “off” periods when this medication does not work well, and L-DOPA treatment can also trigger uncontrolled involuntary movement, a condition called dyskinesia. deep brain stimulation (DBS) can offer symptomatic relief in later stages of PD and may reduce requirements for L-DOPA treatment and exposure to its side effects. DBS is also used to treat other movement disorders, including essential tremor, which causes involuntary shaking (often in the hands) that worsens during movement, and dystonia, which causes involuntary muscle contractions and slow, repetitive movements or abnormal postures.
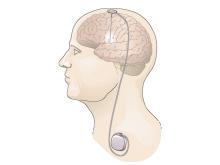
DBS involves a device similar to a cardiac pacemaker that sends electrical signals through wire electrodes implanted in the brain. For movement disorders, electrode locations include brain structures important for motor control. Rigidity, tremor, and dopamine-induced dyskinesia in people with PD are treated with stimulation in the subthalamic nucleus (STN) or the internal segment of the globus pallidus (GPi) (FDA approval, 2002). These same sites are stimulated for the treatment of dystonia (granted a special FDA approval called a Humanitarian Device Exemption (HDE), in 2003). DBS in the ventral intermediate nucleus of the thalamus (VIM) is used to treat essential tremor and tremor as a primary symptom of PD (FDA approval, 1997).
Basic neuroscience research supported by NINDS and NIH to understand neural circuits involved in motor control and how they are affected by PD was essential to the development and clinical application of DBS. NINDS, along with the Department of Veterans Affairs and industry, also sponsored a major clinical trial that showed DBS for PD was superior to L-DOPA treatment alone.
Print Overview and Timeline(pdf, 388 KB)
Timeline
During intraoperative stimulation of potential surgical targets, multiple observations note that high frequency stimulation can mimic the therapeutic effects of lesions4.
Stimulation in the spinal cord and thalamus is used to treat chronic pain, employing modified cardiac pacemaker devices22.

Swiss neurosurgeons Siegfried and Lippitz report that chronic stimulation in the internal segment of the globus pallidus (GPi) improved symptoms in three patients with advanced PD27.
The FDA expands indications for DBS, approving its use in the STN or GPi in advanced PD31.

The FDA allows DBS for dystonia under a Humanitarian Device Exemption (HDE)32.
A large NINDS-funded clinical trial in coordination with the Department of Veterans Affairs shows that DBS is superior to L-DOPA, the best medical therapy at the time, for decreasing PD symptoms and improving motor function33.
Benabid and DeLong share the 2014 Lasker-DeBakey Clinical Medical Research Award for their roles in developing DBS of the STN for the treatment of PD35.

History of Development
Research to understand the brain circuitry underlying movement-related symptoms of PD and other disorders contributed significantly to the development of DBS and can be traced back several decades.
Early surgical treatments
In the 1950s, various movement disorders including PD, essential tremor, and dystonia, were treated by surgically inactivating, or lesioning, brain regions involved in motor control. Surgeons used a frame called a stereotactic apparatus1, which had been developed in 1947, to target specific areas based on knowledge of brain anatomy and function at the time and refined through trial and error. Surgical targets for PD and essential tremor included parts of a central brain structure called the thalamus as well as the globus pallidus (GP), which was serendipitously identified as an effective target when it was damaged by accident during a procedure to lesion a different area2,3. Overall, surgical lesions improved motor symptoms for many patients, though sometimes at the expense of irreversible deficits in other functions.
Neurosurgeons performing these operations used electrodes for brain stimulation in awake patients to define the region to inactivate and limit unintended side effects. As early as the 1960s, a number of reports noted that high frequency stimulation of target regions tended to mimic surgical lesions, while lower frequency stimulation tended to make motor symptoms worse4. Neurophysiologist Natalia Bechtereva of the former Soviet Union was among the first to suggest in the 1970s, that brain stimulation might itself be used as a treatment for movement disorders, instead of permanent lesions4,5. However, another decade would pass before technological advances would make chronic stimulation suitable for broad clinical application. Moreover, medical treatment with L-DOPA6 soon eclipsed neurosurgery as the preferred therapy for PD.
Modeling brain circuits for movement
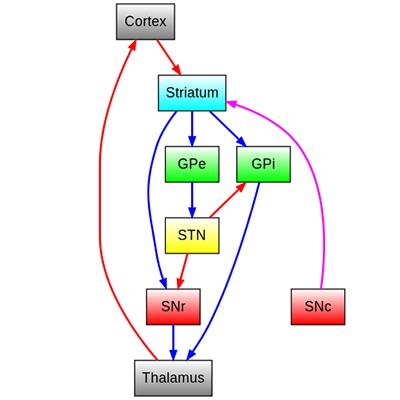
Although interest in ablative surgery for PD waned, researchers were learning more about brain circuits for motor control through studies in animal models. In the 1970s, Mahlon DeLong, then a research fellow at NIH working with scientists at NIMH and NINDS, used electrical stimulation to meticulously characterize the functions of neurons in different brain areas as animals performed movements7,8,9,10. Over a period of several years and continuing after DeLong moved to Johns Hopkins University School of Medicine, these NIH and NINDS-supported studies led to a model of basal ganglia circuit organization, with parallel, functionally segregated circuits involved in movement and other complex functions11,12. As part of the movement-related circuit, the subthalamic nucleus (STN) modulates the GP, which sends signals through the thalamus to the supplementary motor area (SMA), a brain area important for planning and coordinating movement. By this time, researchers knew that the loss of dopamine-producing neurons in the basal ganglia contributed to PD, but how such changes disrupted motor circuit activity and function was still not clear, in part because no animal model of the disease was available.
That changed in the 1980s, after reports described how a few people succumbed to Parkinson’s-like symptoms after taking an illicit drug. NIH scientists published an initial case13, and investigations into more cases by J. William Langston and colleagues in California14 determined that the cause was an impurity known as MPTP (1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine), which selectively killed some of the same neurons lost in PD. Out of this unfortunate event came the opportunity to develop an MPTP-induced animal model of PD, described by NIH scientists15 in 1983. DeLong and others used this model to understand how the disease leads to motor symptoms and to probe the effects of inactivating parts of the basal ganglia circuit16,17. Guided by the circuitry map he helped define, DeLong lesioned the STN in MPTP-treated monkeys and achieved dramatic results: a rapid reduction in PD-like motor symptoms, including tremor, rigidity, and reduced voluntary movement18. Studies by others produced similar results, using either surgical lesions or high frequency stimulation in the STN19,20. Together, the findings altered prevailing assumptions about how circuit abnormalities might drive motor symptoms, and importantly, provided scientific rationale for therapy targeting the STN.
Meanwhile, medical device technology had advanced rapidly in the years since L-DOPA’s introduction, and improved electrical pulse generators had developed alongside the first implantable cardiac pacemakers. The young device company Medtronic began a neurological division21 in 1975, building on early uses of modified pacemakers for neurological indications. For example, NINDS-supported investigators were among the first to use an implanted device for deep brain stimulation (DBS) in the thalamus as a treatment for chronic pain22. Around this time, the limitations of L-DOPA were becoming apparent, and as neurosurgeons sought to improve on past surgical approaches for PD and other motor disorders, some also began exploring the use of DBS.
DBS becomes a clinical success
During a series of surgeries to treat patients with PD and other motor disorders who were not helped by L-DOPA, French neurosurgeon Alim-Louis Benabid observed that applying high frequency stimulation to the ventral intermediate nucleus of the thalamus (VIM) caused the patients’ tremor to cease23. Additional small trials24,25 and Medtronic-funded multisite trials in the US and Europe confirmed the treatment’s effectiveness and showed that thalamic stimulation was safer than surgical inactivation. In 1997, the FDA granted approval for Medtronic’s device for VIM-DBS to treat essential tremor and tremor associated with PD26.
While DBS in the thalamus reduced tremor, it did not reverse other symptoms reported by patients as even more debilitating, including rigidity and slowed or reduced voluntary movement, also called akinesia. Neurosurgeons therefore began to try DBS in other areas. In 1994, Swiss neurosurgeons Jean Siegfried and Bodo Lippitz reported that chronic stimulation in the internal segment of the GP (GPi) improved symptoms in three patients with advanced PD27. Prompted by DeLong’s basal ganglia circuit models and observations on inactivating the STN in MPTP-treated monkeys, Benabid’s team applied DBS to the STN in patients with PD. Stimulating the STN improved tremor, rigidity, and akinesia, yielding their best results in patients yet28,29, and successfully bridging basic and clinical neuroscience research. Based on results from a large, multisite clinical trial30, the FDA approved DBS in the STN or GPi to treat motor symptoms in advanced PD in 200231. A year later, the FDA granted a Humanitarian Device Exemption (HDE) allowing the use of DBS in the GPi and STN for dystonia32.
Subsequent studies have continued to assess long term outcomes of DBS, optimize targets for different symptom profiles, and compare the effectiveness of DBS with medical treatment. In 2009, a pivotal clinical trial jointly supported by NINDS, the Department of Veterans Affairs, and Medtronic demonstrated that DBS for PD was superior to treatment with L-DOPA33. With growing evidence for the safety of DBS and results suggesting earlier intervention in PD may be beneficial, the FDA expanded approval beyond advanced stages of PD in 2016, to allow treatment in patients diagnosed for at least four years who experience troublesome “off” periods or dyskinesia34.
In recognition of their pioneering work to develop DBS for PD, Benabid and DeLong shared the 2014 Lasker-Debakey Clinical Medical Research Award35. Besides motor control, DeLong’s work to understand the functional organization of the basal ganglia also pointed to separate parallel circuits involved in cognitive and emotional functions. Accordingly, researchers are examining the use of DBS to alter basal ganglia circuit activity to treat psychiatric disorders, such as obsessive-compulsive disorder, for which the FDA approved DBS through an HDE in 200936. DBS devices that target other brain areas are also approved and under development for additional neurological disorders, including epilepsy and chronic pain. Moreover, further innovations are emerging with advances in neuroscience and technology. For example, while traditional DBS delivers constant stimulation, newer adaptive devices can self-tune stimulation in response to certain features of a person’s brain activity or behavior. One such closed-loop device is approved for the treatment of medically refractory epilepsy, and research is underway to apply this approach to DBS for PD and other disorders as well37. More broadly, questions remain about exactly how DBS works, and new directions are likely to emerge through research on the mechanisms that underlie its benefits.
List of References
Spiegel EA, Wycis HT, Marks M, Lee AJ. Stereotaxic Apparatus for Operations on the Human Brain. Science. 1947 Oct 10;106(2754):349-50. PMID: 17777432 (American Medical Association)
Cooper IS. Ligation of the anterior choroidal artery for involuntary movements; parkinsonism. Psychiatr Q. 1953 Apr;27(2):317-9. PMID: 13056063
Goetz CG. The history of Parkinson's disease: early clinical descriptions and neurological therapies. Cold Spring Harb Perspect Med. 2011 Sep;1(1):a008862. PMID: 22229124 (review)
Hariz MI, Blomstedt P, Zrinzo L. Deep brain stimulation between 1947 and 1987: the untold story. Neurosurg Focus. 2010 Aug;29(2):E1. PMID: 20672911 (review)
Bekhtereva NP, Bondarchuk AN, Smirnov VM, Meliucheva LA. [Therapeutic electric stimulation of deep brain structures]. Vopr Neirokhir. 1972 Jan-Feb;36(1):7-12. Russian. PMID: 5013728
Show More
Cotzias GC, Papavasiliou PS, Gellene R. Modification of Parkinsonism--chronic treatment with L-dopa. N Engl J Med. 1969 Feb 13;280(7):337-45. PMID: 4178641 (United States Atomic Energy Commission; NIH, grant UI 00421–05)
DeLong MR. Activity of pallidal neurons during movement. J Neurophysiol. 1971 May;34(3):414-27. PMID: 4997823 (NIH/NIMH, intramural)
DeLong MR. Activity of basal ganglia neurons during movement. Brain Res. 1972 May 12;40(1):127-35. PMID: 4624486 (NIH/NIMH, intramural)
DeLong MR. Putamen: activity of single units during slow and rapid arm movements. Science. 1973 Mar 23;179(4079):1240-2. PMID: 4631890 (NIH/NIMH, intramural)
DeLong MR, Strick PL. Relation of basal ganglia, cerebellum, and motor cortex units to ramp and ballistic limb movements. Brain Res. 1974 May 17;71(2-3):327-35. PMID: 4219745 (NIH/NIMH, intramural)
DeLong MR, Georgopoulos AP, Crutcher MD, Mitchell SJ, Richardson RT, Alexander GE. Functional organization of the basal ganglia: contributions of single-cell recording studies. Ciba Found Symp. 1984;107:64-82. PMID: 6389041 (review)
Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357-81. PMID: 3085570 (review; NIH/NINDS, grants NS00632, NS17678, NS16375, NS02957; VA; private donations)
Davis GC, Williams AC, Markey SP, Ebert MH, Caine ED, Reichert CM, Kopin IJ. Chronic Parkinsonism secondary to intravenous injection of meperidine analogues. Psychiatry Res. 1979 Dec;1(3):249-54. PMID: 298352 (NIH, NINDS/NIMH intramural)
Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983 Feb 25;219(4587):979-80. PMID: 6823561 (supported by local government)
Burns RS, Chiueh CC, Markey SP, Ebert MH, Jacobowitz DM, Kopin IJ. A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4546-50. PMID: 6192438 (NIH/NIMH intramural)
Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989 Oct;12(10):366-75. Review. PMID: 2479133 (NIH/NINDS, grants NS15655, NS19613, NS01300; private donations)
DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990 Jul;13(7):281-5. Review. PMID: 1695404 (NIH/NINDS, grant NS015417)
Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990 Sep 21;249(4975):1436-8. PMID: 2402638 (NIH/NINDS, grant NS15417; private funds)
Aziz TZ, Peggs D, Sambrook MA, Crossman AR. Lesion of the subthalamic nucleus for the alleviation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism in the primate. Mov Disord. 1991;6(4):288-92. PMID: 1758446 (UK)
Benazzouz A, Gross C, Féger J, Boraud T, Bioulac B. Reversal of rigidity and improvement in motor performance by subthalamic high-frequency stimulation in MPTP-treated monkeys. Eur J Neurosci. 1993 Apr 1;5(4):382-9. PMID: 8261116 (French government)
Medtronic. (June 2010). A legacy of Innovation: The Medtronic Story [Brochure]. Retrieved from http://wwwp.medtronic.com/newsroom/content/1281109242470.pdf.
Hosobuchi Y, Adams JE, Rutkin B. Chronic thalamic stimulation for the control of facial anesthesia dolorosa. Arch Neurol. 1973 Sep;29(3):158-61. PMID: 4591720 (NIH/NINDS, grant NS5593; private funds)
Benabid AL, Pollak P, Louveau A, Henry S, de Rougemont J. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol. 1987;50(1-6):344-6. PMID: 3329873 (France)
Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, Perret JE, de Rougemont J. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991 Feb 16;337(8738):403-6. PMID: 1671433 (French government)
Blond S, Siegfried J. Thalamic stimulation for the treatment of tremor and other movement disorders. Acta Neurochir Suppl (Wien). 1991;52:109-11. PMID: 1792949 (France)
FDA. Premarket Approval (PMA) Database. Medtronic Activa Tremor Control System. Retrieved from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm?id=P960009.
Siegfried J, Lippitz B. Bilateral chronic electrostimulation of ventroposterolateral pallidum: a new therapeutic approach for alleviating all parkinsonian symptoms. Neurosurgery. 1994 Dec;35(6):1126-9; discussion 1129-30. PMID: 7885558 (Switzerland)
Limousin P, Pollak P, Benazzouz A, Hoffmann D, Le Bas JF, Broussolle E, Perret JE, Benabid AL. Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet. 1995 Jan 14;345(8942):91-5. PMID: 7815888 (French government)
Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, Benabid AL. Electrical stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 1998 Oct 15;339(16):1105-11. PMID: 9770557 (French Government; Medtronic)
Deep-Brain Stimulation for Parkinson's Disease Study Group, Obeso JA, Olanow CW, Rodriguez-Oroz MC, Krack P, Kumar R, Lang AE. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson's disease. N Engl J Med. 2001 Sep 27;345(13):956-63. PMID: 11575287 (Medtronic)
FDA. Premarket Approval (PMA) Database. Medtronic Activa Parkinson’s Control System. Retrieved from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P960009S007.
FDA. Humanitarian Device Exemption (HDE) Database. Medtronic Activa Deep Brain Stimulation (DBS) System. Retrieved from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfhde/hde.cfm?id=375511.
Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ Jr, Rothlind J, Sagher O, Reda D, Moy CS, Pahwa R, Burchiel K, Hogarth P, Lai EC, Duda JE, Holloway K, Samii A, Horn S, Bronstein J, Stoner G, Heemskerk J, Huang GD; CSP 468 Study Group. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009 Jan 7;301(1):63-73. PMID: 19126811 (NINDS, VA, Medtronic)
Medtronic. (February 17, 2016). FDA Approves Medtronic Deep Brain Stimulation for People with Parkinson's Disease with Recent Onset of Motor Complications [Press Release]. Retrieved from http://newsroom.medtronic.com/phoenix.zhtml?c=251324&p=irol-newsArticle&ID=2139957.
Albert and Mary Lasker Foundation. 2014 Lasker~DeBakey Clinical Medical Research Award. Retrieved from http://www.laskerfoundation.org/awards/show/deep-brain-stimulation-for-parkinsons-disease/.
FDA. Humanitarian Device Exemption (HDE) Database. Medtronic Activa Deep Brain Stimulation (DBS) for OCD Therapy. Retrieved from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfhde/hde.cfm?id=375533.
National Institute of Neurological Disorders and Stroke. (May 29, 2018.) Self-tuning brain implant could help treat patients with Parkinson’s disease. Retrieved from https://www.ninds.nih.gov/News-Events/News-and-Press-Releases/Press-Releases/Self-tuning-brain-implant-could-help-treat.