Overview
Epilepsy is a chronic neurological condition characterized by recurring seizures, or abnormal bursts of electrical activity in the brain that can trigger jerky movements, strange sensations or emotions, unusual behavior, and/or loss of consciousness. Although available anti-seizure medications help the majority of people with epilepsy, a third or more receive little or no relief from medical treatment. Some people with medically refractory epilepsy experience excellent outcomes with surgery to remove or disconnect brain tissue that initiates or propagates seizures, but for others, a clear seizure focus may be difficult to identify or target safely without causing new neurological deficits. In these cases, neurostimulation devices can offer another alternative.
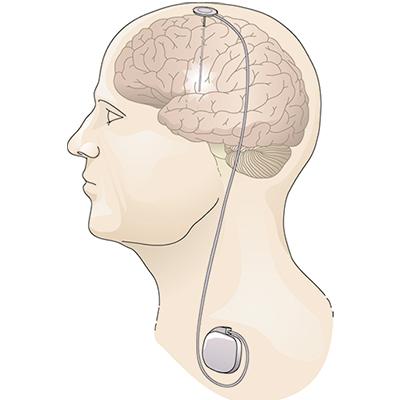
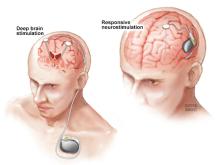
Research supported by NINDS and conducted by NINDS scientists figured prominently in the development of two FDA-approved devices that deliver electrical stimulation to the brain in different ways to reduce seizure frequency in people who do not achieve good seizure control with medication alone. Deep brain stimulation (DBS) for epilepsy, approved in 2018, delivers chronic stimulation to the anterior nucleus of the thalamus (ANT), a small brain structure involved in the spread of an initially localized seizure. In contrast, responsive neurostimulation (RNS®), approved in 2013, delivers stimulation directly to the source of an individual’s seizures, but only when continuously monitored brain activity suggests a seizure may be beginning.
Print Overview and Timeline(pdf, 745 KB)
Timeline
During recordings of brain activity in people with epilepsy undergoing surgery, Wilder Penfield and Herbert Jasper observe that seizure-like activity could be halted with brief counter stimulation1.

Low frequency stimulation in the anterior nucleus of the thalamus (ANT) is reported to synchronize brain activity, while high frequency stimulation had the opposite effect9.
The first systems emerge for automated seizure detection based on EEG recordings of brain activity.30
High frequency stimulation in the ANT is shown to protect against generalized seizures in an animal model.14
The pivotal clinical trial of the NeuroPace responsive neurostimulation system (RNS®) shows that seizure frequency decreased in individuals receiving stimulation39.
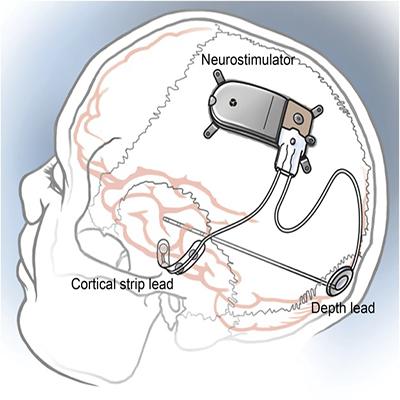
The NeuroPace RNS® receives approval from the FDA for use in adults with medically refractory focal epilepsy40.

Medtronic’s DBS System for Epilepsy receives approval from the FDA for use in adults with medically refractory focal epilepsy24.

History of Development
Research supported by NINDS and conducted by NINDS scientists proved critical to the development of the first brain stimulation therapies for epilepsy, including studies to rigorously test early approaches, find new stimulation targets by understanding seizure mechanisms, and develop algorithms for automated seizure detection.
Pioneering neurosurgeons set the stage
Neurostimulation therapies for epilepsy follow a longer history of attempts to quiet seizures through surgical interventions. In the 1930s, Canadian neurosurgeon Wilder Penfield pioneered modern methods for surgery to treat epilepsy. He used electroencephalography (EEG) to identify where seizures originated in the brain of individuals with epilepsy and then more finely mapped targets for surgical resection with direct electrical stimulation through electrodes inserted into the brain. This basic approach to surgical evaluation in epilepsy remains in practice today, with ongoing improvements as brain imaging and recording technologies have advanced.
Beyond improving the success of surgery for people with epilepsy, intraoperative recording also provided new insights about how seizures originate and propagate in the brain and laid the foundation for therapeutic stimulation. Indeed, Penfield and colleague Herbert Jasper were among the first to observe that electrical stimulation to the cortex could silence seizure discharges1. By the 1960s, with the advent of the cardiac pacemaker and the exploration of deep brain stimulation (DBS) therapies to treat pain and motor disorders, researchers began to pursue similar treatments for epilepsy. The choice of targets for stimulation were based in part on research using electrical stimulation to understand seizure mechanisms in animal models. For example, in the 1970s, neurosurgeon Irving Cooper hypothesized that stimulation in the cerebellum could reduce seizures in people with epilepsy, a suggestion supported by NIH-funded studies in cats2,3. Although Cooper’s initial trials were promising4,5, placebo-controlled trials and animal studies conducted by NINDS intramural scientists and others failed to confirm the benefit of treatment6,7,8. Nevertheless, these early efforts were important for demonstrating the feasibility and safety of brain stimulation for epilepsy, and for establishing the need to control for a strong placebo effect and for changes related to surgery alone.
Identifying the anterior nucleus of the thalamus as a target for seizure control
In the search for other targets, several lines of evidence pointed to a small brain region called the anterior nucleus of the thalamus (ANT). Low frequency stimulation in this region was reported to synchronize brain activity, while high frequency stimulation had the opposite effect, suggesting it may also disrupt seizures9. NIH-supported investigators found that surgical lesions of the ANT decreased the occurrence and duration of seizures in a small clinical study10 and in an animal model11. A series of NINDS-funded studies on the mechanisms of experimentally induced seizures placed the ANT within a brain circuit that mediates seizure generalization, in which seizure activity initiated in a small focused area (focal seizure) spreads to larger areas of the brain12,13. A subsequent study prompted by these findings showed that high-frequency stimulation in the ANT could protect against generalized seizures14. Altogether, the ANT’s anatomically defined position and its role in seizure spread from other locations made it an appealing therapeutic target, potentially for a wide range of individuals with seizures initially originating in different areas.
In the 1980s, Cooper reported that chronic ANT stimulation improved seizure control in small numbers of people with medically refractory epilepsy15,16,17. While promising, these studies were unblinded and uncontrolled, and the use of an external stimulator was impractical. A decade later, the FDA had approved two implantable neurostimulation devices, Medtronic’s deep brain stimulation (DBS) system as a treatment for tremor18 and Cyberonics’ vagal nerve stimulator for medically refractory epilepsy19. These approvals helped renew interest in further development of DBS for epilepsy, and in the early 2000s, preliminary studies assessed the use of Medtronic’s DBS system for bilateral ANT stimulation20,21, including one study conducted by investigators with NINDS and NIMH support19. Medtronic subsequently sponsored a randomized, controlled clinical trial 110 patients. The SANTE (Stimulation of the Anterior Nucleus of the Thalamus in Epilepsy) trial and a seven-year follow up period23 showed lasting benefits of treatment in terms of reduced seizure frequency and meaningful improvements in quality of life. These results led the FDA to approve Medtronic’s DBS System for Epilepsy in 2018, for use along with medical treatment in adults with focal epilepsy who have not achieved seizure control after trying three or more epilepsy medications24.
Responsive neurostimulation to halt seizures at their source
The DBS system for epilepsy delivers ongoing, or open loop, stimulation intended to alter brain activity just enough to prevent or limit the initiation or spread of a seizure. In contrast, closed loop or responsive stimulation, delivers stimulation only when brain activity patterns suggest a seizure is beginning or very likely to occur. Beyond Jasper and Penfield’s original observations1 in 1954, later studies–in people undergoing surgery for epilepsy and in animal models with experimentally induced seizures–provided a rationale for this approach. This research supported by NINDS and others showed that brief bursts of stimulation could terminate seizures or seizure-like activity when delivered soon after onset, either to brain structures remote from seizure activity25,26, or directly to the onset site27,28,29. For example, brain stimulation used to map an individual’s seizure focus for surgery sometimes elicits rhythmic activity called afterdischarges that resemble spontaneous seizure activity. In 1999, NINDS-supported investigators reported that brief counter stimulation at the same site could terminate such afterdischarges28.
In parallel, an expanding area of research sought to develop algorithms for automated seizure detection. By analyzing brain activity data from people with epilepsy, investigators worked to identify features that reliably indicated a seizure was beginning or very likely to occur. Early seizure detection systems emerged30 around the 1980s, and by the late 1990s, academic and industry investigators supported by NINDS, NIMH, and private sources were developing more refined algorithms that would ultimately be adapted for use in the first responsive stimulation devices31,32,33,34. Initial tests for safety and feasibility used prototypes with non-implantable external neurostimulators in individuals with epilepsy undergoing evaluation for surgery35,36,37. After promising preliminary results38, the device company NeuroPace was the first to launch a large, randomized, multi-center trial for their implantable responsive neurostimulation system39. The pivotal trial showed that seizure frequency decreased in study participants receiving stimulation compared to those in a control group who received a sham procedure. Based on these findings, the NeuroPace RNS® was approved by the FDA in 2013, for use along with medical treatment in adults with medically refractory focal epilepsy40. A key feature of the RNS® system is the ability for physicians to fine-tune detection and stimulation parameters for each person, possibly improving outcomes over time.
Future directions in neurostimulation and responsive therapies for epilepsy
Research continues to improve neurostimulation therapies for epilepsy. Efforts are underway to incorporate seizure forecasting and responsive stimulation into DBS for epilepsy, including a project supported through the NIH BRAIN Initiative Public-Private Partnership Program to facilitate clinical research using industry-supplied cutting-edge devices41. In addition, there is hope that future algorithms for triggering responsive epilepsy therapies will more accurately detect or even predict seizures, allowing advanced warning for patients and potentially better seizure control. In 2014, NINDS, the American Epilepsy Society, and the Epilepsy Foundation launched a crowd-sourced seizure detection and prediction challenge in which amateur computer programmers, scientists, and others from around the world competed to develop algorithms tested on long-term intracranial EEG data42. The best algorithms significantly increased seizure prediction accuracy over prior methods, a success that bolstered interest in further innovation: a second international contest was held43 in 2016, and an online portal provides access to datasets for ongoing algorithm development.
List of References
Penfield W, Jasper H. Electrocorticography. Epilepsy and the functional anatomy of the human brain. Boston, MA: Little, Brown; 1954. pp. 692–738.
Cooke PM, Snider RS. Some cerebellar influences on electrically-induced cerebral seizures. Epilepsia. 1955 Nov;4:19-28. PMID: 13305547 (Office of Naval Research and the U.S. Public Health Service/NIH)
Dow RS, Fernandez-Guardiola A, Manni E. The influence of the cerebellum on experimental epilepsy. Electroencephalogr Clin Neurophysiol. 1962 Jun;14:383-98.PMID: 13887605 (NIH/NINDS, grant B-2289)
Cooper IS, Amin I, Gilman S. The effect of chronic cerebellar stimulation upon epilepsy in man. Trans Am Neurol Assoc. 1973;98:192-6. PMID: 4206369 (funding source not stated)
Cooper IS, Amin I, Riklan M, Waltz JM, Poon TP. Chronic cerebellar stimulation in epilepsy. Clinical and anatomical studies. Arch Neurol. 1976 Aug;33(8):559-70. PMID: 821458 (Private support)
Show More
Van Buren JM, Wood JH, Oakley J, Hambrecht F. Preliminary evaluation of cerebellar stimulation by double-blind stimulation and biological criteria in the treatment of epilepsy. J Neurosurg. 1978 Mar;48(3):407-16. PMID: 344840 (NIH/NINDS, intramural)
Strain GM, Babb TL, Soper HV, Perryman KM, Lieb JP, Crandall PH. Effects of chronic cerebellar stimulation on chronic limbic seizures in monkeys. Epilepsia. 1979 Dec;20(6):651-64. PMID: 115677 (NIH/NINDS, contract NS 4-2331)
Wright GD, McLellan DL, Brice JG. A double-blind trial of chronic cerebellar stimulation in twelve patients with severe epilepsy. J Neurol Neurosurg Psychiatry. 1984 Aug;47(8):769-74. PMID: 6381652 (UK, Medical Research Council)
Jasper H, Naquet R, KingEV. Thalamocortical recruiting responses in sensory receiving areas in the cat. Electroencephalogr Clin Neurophysiol. 1955 Feb;7(1):99-114. PMID: 14353024 (Rockefeller Foundation)
Mullan S, Vailati G, Karasick J, Mailis M. Thalamic lesions for the control of epilepsy. A study of nine cases. Arch Neurol. 1967 Mar;16(3):277-85. PMID:4959933 (NIH grant; Brain Research Foundation)
Kusske JA, Ojemann GA, Ward AA Jr. Effects of lesions in ventral anterior thalamus on experimental focal epilepsy. Exp Neurol. 1972 Feb;34(2):279-90. PMID: 4622698 (NIH/NINDS, grants NS05211, NS04053; Moy Mill Foundation)
Mirski MA, Ferrendelli JA. Interruption of the mammillothalamic tract prevents seizures in guinea pigs. Science. 1984 Oct 5;226(4670):72-4. PMID: 6433485 (NIH/NINDS, grant NS14834; Seay Neuropharmacology Research Fellowship)
Mirski MA, Ferrendelli JA. Anterior thalamic mediation of generalized pentylenetetrazol seizures. Brain Res. 1986 Dec 10;399(2):212-23. PMID: 3548879 (NIH Grants NS14834 (Ferrendelli, Washington University program project on the basic mechanisms of epilepsy) and GM07200 (T32, medical scientist) and the Seay Neuropharmacology Research Fellowship)
Mirski MA, Rossell LA, Terry JB, Fisher RS. Anticonvulsant effect of anterior thalamic high frequency electrical stimulation in the rat. Epilepsy Res. 1997 Sep;28(2):89-100. PMID: 9267773 (funding source not stated)
Cooper IS, Upton AR, Amin I. Reversibility of chronic neurologic deficits. Some effects of electrical stimulation of the thalamus and internal capsule in man. Appl Neurophysiol. 1980;43(3-5):244-58. PMID: 6975064 (John A. Hartford Foundation)
Upton AR, Cooper IS, Springman M, Amin I. Suppression of seizures and psychosis of limbic system origin by chronic stimulation of anterior nucleus of the thalamus. Int J Neurol. 1985-1986;19-20:223-30. PMID: 2980675
Upton AR, Amin I, Garnett S, Springman M, Nahmias C, Cooper IS. Evoked metabolic responses in the limbic-striate system produced by stimulation of anterior thalamic nucleus in man. Pacing Clin Electrophysiol. 1987 Jan;10(1 Pt 2):217-25. PMID: 2436182. (source not provided, Canada)
FDA. Premarket Approval (PMA) Database. Medtronic Activa Tremor Control System. Retrieved from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm?id=P960009.
FDA. Premarket Approval (PMA) Database. VNS Therapy System. Retrieved from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P970003.
Hodaie M, Wennberg RA, Dostrovsky JO, Lozano AM. Chronic anterior thalamus stimulation for intractable epilepsy. Epilepsia. 2002 Jun;43(6):603-8. PMID: 12060019 (Canada, private sources; Medtronic equipment)
Kerrigan JF, Litt B, Fisher RS, Cranstoun S, French JA, Blum DE, Dichter M, Shetter A, Baltuch G, Jaggi J, Krone S, Brodie M, Rise M, Graves N. Electrical stimulation of the anterior nucleus of the thalamus for the treatment of intractable epilepsy. Epilepsia. 2004 Apr;45(4):346-54. PMID: 15030497 (NIH/NINDS, grants NS041811 and NS044001; NIH/NIMH, grant MH062298; Whitaker Foundation, American Epilepsy Society, Charles Henry Dana Foundation, Sandra Solheim Aiken Fund for Epilepsy, U.S. Veterans Administration; clinical costs including implantable devices provided by Medtronic, Inc)
Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, Oommen K, Osorio I, Nazzaro J, Labar D, Kaplitt M, Sperling M, Sandok E, Neal J, Handforth A, Stern J, DeSalles A, Chung S, Shetter A, Bergen D, Bakay R, Henderson J, French J, Baltuch G, Rosenfeld W, Youkilis A, Marks W, Garcia P, Barbaro N, Fountain N, Bazil C, Goodman R, McKhann G, Babu Krishnamurthy K, Papavassiliou S, Epstein C, Pollard J, Tonder L, Grebin J, Coffey R, Graves N; SANTE Study Group. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010 May;51(5):899-908. Epub 2010 Mar 17. PMID: 20331461. (Medtronic; NIH/NINDS, grant NS044001 [planning only])
Salanova V, Witt T, Worth R, Henry TR, Gross RE, Nazzaro JM, Labar D, Sperling MR, Sharan A, Sandok E, Handforth A, Stern JM, Chung S, Henderson JM, French J, Baltuch G, Rosenfeld WE, Garcia P, Barbaro NM, Fountain NB, Elias WJ, Goodman RR, Pollard JR, Tröster AI, Irwin CP, Lambrecht K, Graves N, Fisher R; SANTE Study Group. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 2015 Mar 10;84(10):1017-25. Epub 2015 Feb 6. PMID: 25663221 (Medtronic)
FDA. Premarket Approval (PMA) Database. Medtronic DBS System for Epilepsy. Retrieved from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P960009S219.
Psatta DM. Control of chronic experimental focal epilepsy by feedback caudatum stimulations. Epilepsia. 1983 Aug;24(4):444-54. PMID: 6603358 (Romania, funding source not stated)
Chkhenkeli SA, Chkhenkeli IS. Effects of therapeutic stimulation of nucleus caudatus on epileptic electrical activity of brain in patients with intractable epilepsy. Stereotact Funct Neurosurg. 1997;69(1-4 Pt 2):221-4. PMID: 9711758 (funding source not stated)
Nakagawa M, Durand D. Suppression of spontaneous epileptiform activity with applied currents. Brain Res. 1991 Dec 20;567(2):241-7. PMID: 1817729 (funding source not stated)
Lesser RP, Kim SH, Beyderman L, Miglioretti DL, Webber WR, Bare M, Cysyk B, Krauss G, Gordon B. Brief bursts of pulse stimulation terminate afterdischarges caused by cortical stimulation. Neurology. 1999 Dec 10;53(9):2073-81. PMID: 10599784 (NS26553, NS29973, private funding)
Motamedi GK, Lesser RP, Miglioretti DL, Mizuno-Matsumoto Y, Gordon B, Webber WR, Jackson DC, Sepkuty JP, Crone NE. Optimizing parameters for terminating cortical afterdischarges with pulse stimulation. Epilepsia. 2002 Aug;43(8):836-46. Erratum in: Epilepsia 2002 Nov;43(11):1441. PMID: 12181002 (funding source not stated)
Gotman J. Automatic recognition of epileptic seizures in the EEG. Electroencephalogr Clin Neurophysiol. 1982 Nov;54(5):530-40. PMID: 6181976 (Medical Research Council of Canada)
Osorio I, Frei MG, Wilkinson SB. Real-time automated detection and quantitative analysis of seizures and short-term prediction of clinical onset. Epilepsia. 1998 Jun;39(6):615-27. PMID: 9637604 (NIH/NINDS, grant NS-34630, Dons Flynn & Hoechst Marrion Roussel research awards)
Osorio I, Frei MG, Giftakis J, Peters T, Ingram J, Turnbull M, Herzog M, Rise MT, Schaffner S, Wennberg RA, Walczak TS, Risinger MW, Ajmone-Marsan C. Performance reassessment of a real-time seizure-detection algorithm on long ECoG series. Epilepsia. 2002 Dec;43(12):1522-35. PMID: 12460255 (NIH/NINDS, grant NS34530-03; Medtronic)
D'Alessandro M, Esteller R, Vachtsevanos G, Hinson A, Echauz J, Litt B. Epileptic seizure prediction using hybrid feature selection over multiple intracranial EEG electrode contacts: a report of four patients. IEEE Trans Biomed Eng. 2003 May;50(5):603-15. Erratum in: IEEE Trans Biomed Eng. 2003 Aug;50(8):1041. PMID: 12769436 (NIH/NINDS, grant NS041811; NIH/NIMH, grant MH62298; Whitaker Foundation, Epilepsy Foundation, American Epilepsy Society, University of Pennsylvania Research Foundation; NeuroPace)
Echauz J, Esteller R, Tcheng T, Pless B, Gibb B, Litt B. Long‐term validation of detection algorithms suitable for an implantable device. Epilepsia 2001;42(suppl 7):35–6. (conference abstract)
Peters TE, Bhavaraju NC, Frei MG, Osorio I. Network system for automated seizure detection and contingent delivery of therapy. J Clin Neurophysiol. 2001 Nov;18(6):545-9. PMID: 11779967 (NIH/NINDS, grant NS34630)
Kossoff EH, Ritzl EK, Politsky JM, Murro AM, Smith JR, Duckrow RB, Spencer DD, Bergey GK. Effect of an external responsive neurostimulator on seizures and electrographic discharges during subdural electrode monitoring. Epilepsia. 2004 Dec;45(12):1560-7. PMID: 15571514 (NeuroPace)
Osorio I, Frei MG, Sunderam S, Giftakis J, Bhavaraju NC, Schaffner SF, Wilkinson SB. Automated seizure abatement in humans using electrical stimulation. Ann Neurol. 2005 Feb;57(2):258-68. PMID: 15668970 (Medtronic; State Small Business Innovation Research grant; Flint Hills Scientific equipment)
Barkley GL, Smith B, Bergey G, Worrell G, Chabolla D, Drazkowski J, Labar D, Duckrow R, Murro A, Smith M, Gwinn R, Fisch B, Hirsch L, and Morrell M. Safety and Preliminary Efficacy of the RNS Responsive Neurostimulator for the Treatment of Intractable Epilepsy in Adults. Epilepsia 2006; 47(S4):5. (conference abstract; ClinicalTrials.gov NCT00079781)
Morrell MJ; RNS System in Epilepsy Study Group. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011 Sep 27;77(13):1295-304. PMID: 21917777 (NeuroPace; ClinicalTrials.gov NCT00264810)
FDA. Premarket Approval (PMA) Database. NeuroPace RNS System. Retrieved from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P100026.
NIH RePORTER. Neurophysiologically Based Brain State Tracking & Modulation in Focal Epilepsy. Retrieved from https://projectreporter.nih.gov/project_info_description.cfm?aid=9531467&icde=40851200.
NINDS. News Articles. Winners announced in NIH-supported crowdsourcing contest of seizure prediction. Retrieved from http://wayback.archiveit.org/1170/20150104175408/http://www.ninds.nih.gov/news_and_events/news_articles/pressrelease_epilepsy_challenge_12162014.htm
Kaggle. Melbourne University AES/MathWorks/NIH Seizure Prediction. Retrieved from https://www.kaggle.com/c/melbourne-university-seizure-prediction#evaluation.