- Organization Chart
- Appropriation Language
- Amounts Available for Obligation
- Budget Mechanism Table
- Major Changes in Budget Request
- Summary of Changes
- Budget Graphs
- Budget Authority by Activity
- Authorizing Legislation
- Appropriations History
- Justification of Budget Request
- Budget Authority by Object Class
- Salaries and Expenses
- Detail of Full-Time Equivalent Employment (FTE)
- Detail of Positions
Organization Chart

National Institutes of Health
National Institute of Neurological Disorders and Stroke
For carrying out section 301 and title IV of the PHS Act with respect to neurological disorders and stroke, [$2,374,687,000]$2,195,110,000.
NIH INNOVATION ACCOUNT, CURES ACT (INCLUDING TRANSFER OF FUNDS)
For necessary expenses to carry out the purposes described in section 1001(b)(4) of the 21st Century Cures Act, in addition to amounts available for such purposes in the appropriations provided to the NIH in this Act, [$492,000,000]$404,000,000, to remain available until expended: Provided, That such amounts are appropriated pursuant to section 1001(b)(3) of such Act, are to be derived from amounts transferred under section 1001(b)(2)(A) of such Act, and may be transferred by the Director of the National Institutes of Health to other accounts of the National Institutes of Health solely for the purposes provided in such Act: Provided further, That upon a determination by the Director that funds transferred pursuant to the previous proviso are not necessary for the purposes provided, such amounts may be transferred back to the Account: Provided further, That the transfer authority provided under this heading is in addition to any other transfer authority provided by law. Department of Health and Human Services Appropriations Act, 2020.
Amounts Available for Obligation 1
(Dollars in Thousands)
| Source of Funding | FY 2019 Final | FY 2020 Enacted | FY 2021 President's Budget |
|---|---|---|---|
| Mandatory Appropriation: (non-add) | |||
| Appropriation2 | $2,274,413 | $2,444,687 | $2,245,110 |
| Type 1 Diabetes | (0) | (0) | (0) |
| Other Mandatory financing | (0) | (0) | (0) |
| Rescission | 0 | 0 | 0 |
| Sequestration | 0 | 0 | 0 |
| Secretary's Transfer | -6,756 | 0 | 0 |
| Subtotal, adjusted appropriation OAR HIV/AIDS Transfers HEAL Transfer | $2,267,657 -4,349 -17,000 | $2,444,687 1,890 0 | $2,245,110 0 0 |
| Subtotal, adjusted budget authority Unobligated balance, start of year Unobligated balance, end of year3 | $2,246,308 170,437 -2,845 | $2,446,577 2,845 0 | $2,245,110 0 0 |
| Subtotal, adjusted budget authority Unobligated balance lapsing | $2,413,900 -3 | $2,449,422 0 | $2,245,110 0 |
| Total obligations | $2,413,897 | $2,449,422 | $2,245,110 |
1 Excludes the following amounts (in thousands) for reimbursable activities carried out by this account:
FY 2019 - $18,957 FY 2020 - $19,700 FY 2021 - $19,560
2 Of which $57.5 million in FY 2019, $70.0 million in FY 2020, and $50.0 million in FY 2021 is derived by transfer from the NIH Innovation Account under the 21st Century Cures Act.
3 Reflects 21st Century Cures Act funding not obligated in FY 2019, and carried over into FY 2020.
Budget Mechanism - Total 1,2
(Dollars in Thousands)
| MECHANISM | FY 2019 Final3 | FY 2020 Enacted | FY 2021 President's Budget | FY 2021 +/- FY 2020 Enacted | ||||
|---|---|---|---|---|---|---|---|---|
| No. | Amount | No. | Amount | No. | Amount | No. | Amount | |
| Research Projects: | ||||||||
| Competing: | ||||||||
Research Centers: | ||||||||
Other Research: | ||||||||
Ruth L. Kirschstein Training Awards: | FTTPs | FTTPs | FTTPs | |||||
| Noncompeting | 2,216 | $994,637 | 2,538 | $1,158,175 | 2,688 | $1,217,796 | 150 | $59,621 |
| Administrative Supplements | (97) | 9,131 | (135) | 28,394 | (59) | 8,842 | (-76) | -19,552 |
| Renewal | 115 | 60,486 | 136 | 70,294 | 87 | 38,866 | -49 | -31,428 |
| New | 756 | 491,005 | 868 | 456,184 | 555 | 288,700 | -313 | -167,484 |
| Supplements | 2 | 347 | 0 | 0 | 0 | 0 | 0 | 0 |
| Subtotal, Competing | 873 | $551,839 | 1,004 | $526,478 | 642 | $327,566 | -362 | -198,912 |
| Subtotal, RPGs | 3,089 | $1,555,607 | 3,542 | $1,713,047 | 3,330 | $1,554,204 | -212 | -158,843 |
| SBIR/STTR | 146 | 80,188 | 140 | 79,730 | 127 | 72,931 | -13 | -6,799 |
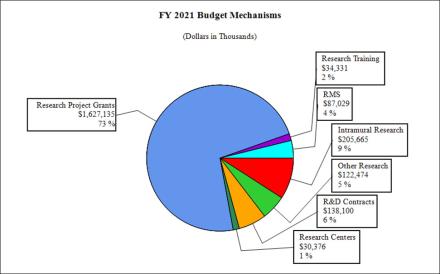
| Research Project Grants | 3,235 | $1,635,795 | 3,682 | $1,792,777 | 3,457 | $1,627,135 | -225 | -$165,642 |
| Specialized/Comprehensive | 36 | $39,575 | 30 | $31,627 | 23 | $30,276 | -7 | -$1,351 |
| Clinical Research | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Biotechnology | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Comparative Medicine | 0 | 100 | 0 | 100 | 0 | 100 | 0 | 0 |
| Research Centers in Minority Institutions | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Research Centers | 36 | $39,675 | 30 | $31,727 | 23 | $30,376 | -7 | $1,351 |
| Research Careers | 226 | $42,015 | $281 | $44,426 | 250 | $42,772 | -31 | -$1,653 |
| Cancer Education | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cooperative Clinical Research | 0 | 0 | 1 | 1,714 | 0 | 0 | -1 | -1,714 |
| Biomedical Research Support | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Minority Biomedical Research Support | 1 | 164 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 237 | 106,691 | 249 | 81,558 | 215 | 79,702 | -34 | -1,856 |
| Other Research | 464 | $148,870 | 531 | $127,698 | 465 | $122,474 | -66 | -$5,224 |
| Total Research Grants | 3,735 | $1,824,340 | 4,243 | $1,952,202 | 3,945 | $1,779,985 | -298 | -$172,217 |
| Individual Awards | 374 | 16,633 | 389 | $17,982 | 365 | $18,136 | -24 | -$154 |
| Institutional Awards | 283 | 15,275 | 372 | 17,047 | 330 | 16,195 | -42 | -853 |
| Total Research Training | 657 | $31,923 | 689 | $35,030 | 695 | $34,331 | -66 | -$699 |
Research & Development Contracts | 110 | $97,110 | 106 | $148,544 | 115 | $138,100 | -5 | -$10,444 |
| SBIR/STTR (non-add) | (6) | (141) | (2) | (970) | (3) | (968) | (-3) | (-2) |
| Intramural Research | 287 | 197,036 | 318 | $219,577 | 307 | 205,665 | 0 | -13,912 |
| Research Management and Support | 209 | 82,307 | 225 | 91,224 | 225 | 87,029 | 0 | -4,195 |
| Res. Management & Support (SBIR Admin) (non-add) | (0) | (358) | (0) | (372) | (0) | (372) | (0) | (0) |
| Construction | 0 | 0 | 0 | 0 | ||||
| Buildings and Facilities | 0 | 0 | 0 | 0 | ||||
| Total, NINDS | 496 | $2,246,308 | 532 | $2,446,577 | 532 | $2,245,110 | 0 | -$201,467 |
1 All items in italics and brackets are non-add entries.
2 Of which $57.5 million in FY 2019, $70.0 million in FY 2020, and $50.0 million in FY 2021 is derived by transfer from the NIH Innovation Account under the 21st Century Cures Act.
3 Includes $2.8 million of 21st Century Cures Act funding not obligated in FY 2019 and carried over into FY 2020.
Major Changes in the Fiscal Year 2020 President's Budget Request
Major changes by budget mechanism and/or budget activity detail are briefly described below. Note that there may be overlap between budget mechanism and activity detail, and these highlights will not sum to the total change for the FY 2021 President’s Budget request for the National Institute of Neurological Disorders and Stroke (NINDS), which is $2,245.1 million, a decrease of $201.5 million from the FY 2020 Enacted level. The FY 2021 President's Budget reflects the Administration's fiscal policy goals for the Federal Government. Within that framework, NINDS will pursue its highest research priorities through strategic investments and careful stewardship of appropriated funds.
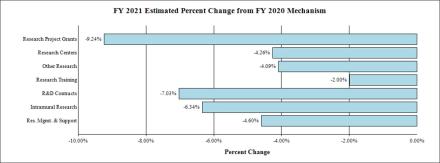
Research Project Grants (RPGs) (-$165.6 million; total $1,627.1 million):
The NINDS budget mechanism table reflects a decrease in $165.6 million in the Institute’s Research Project Grants portfolio. NINDS plans a reduction of 7.0 percent in the cost of individual noncompeting awards relative to their committed level. Competing RPGs are expected to decrease by 362 grants in FY 2021 compared to the FY 2020 enacted level of awards.
Other Research (-$5.2 million; total $122.5 million):
NINDS plans to reduce its regular portfolio of Other Research. This mechanism also reflects an increase in Other Research grants funded from Opioid funding and from the BRAIN Initiative under the 21st> Century Cures Act.
Intramural Research (-$13.9 million; total $205.7 million):
The intramural budget mechanism reflects a 6.3 percent decrease from the funding level of FY 2020.
Summary of Changes
(Dollars in Thousands)
| FY 2020 Enacted | $2,446,577 |
|---|---|
| F Y 2021 President's Budget | $2,245,110 |
| Net change | -$201,467 |
| 2021 President's Budget | Change from FY 2020 Enacted | |||
|---|---|---|---|---|
| CHANGES | FTE's | Budget Authority | FTE's | Budget Authority |
| A. Built-in: 1. Intramural research: | ||||
2. Research Management and Support: | ||||
| a. Annualization of January 2020 pay increase & benefits | $62,370 | $405 | ||
| b. January FY 2021 pay increase & benefits | 62,370 | 925 | ||
| c. Paid days adjustment | 62,370 | -233 | ||
| d. Differences attributable to change in FTE | 62,370 | 0 | ||
| e. Payment for centrally furnished services | 30,848 | -1,624 | ||
| f. Cost of laboratory supplies, materials, other expenses,and non-recurring costs | 112,447 | -200 | ||
| Subtotal | -$727 | |||
| a. Annualization of January 2020 pay increase &benefits | $40,947 | $265 | ||
| b. January FY 2021 pay increase & benefits | 40,947 | 604 | ||
| c. Paid days adjustment | 40,947 | -153 | ||
| d. Differences attributable to change in FTE | 40,947 | 0 | ||
| e. Payment for centrally furnished services | 6,658 | -350 | ||
| f. Cost of laboratory supplies, materials, other expenses, and non-recurring costs | 39,424 | -195 | ||
| Subtotal | $171 | |||
| Subtotal, Built-in | -$556 | |||
| 2021 President's Budget | Change from FY 2020 Enacted | |||
|---|---|---|---|---|
| CHANGES | No. | Amount | No. | Amount |
| B. Program: | ||||
| 1. Research Project Grants: | ||||
| a. Noncompeting | 2,688 | $1,226,638 | 150 | $40,069 |
| b. Competing | 642 | 327,566 | -362 | -198,912 |
| c. SBIR/STTR | 127 | 72,931 | -13 | -6,799 |
| Subtotal, RPGs | 3,457 | $1,627,135 | -225 | -$165,642 |
| 2. Research Centers | 23 | $30,376 | -7 | $1,351 |
| 3. Other Research | 465 | 122,474 | -66 | -5,224 |
| 4. Research Training | 695 | 34,331 | -66 | -699 |
| 5. Research and Development Contracts | 115 | 138,100 | -5 | -10,444 |
| Subtotal, Extramural | $1,952,416 | -$183,360 | ||
| FTE's | FTE's | |||
| 6. Intramural Research | 307 | $205,665 | 0 | -$13,186 |
| 7. Research Management and Support | 225 | 87,029 | 0 | -4,366 |
| 8. Construction | 0 | 0 | ||
| 9. Buildings and Facilities | 0 | 0 | ||
| Subtotal, Program | 532 | $2,245,110 | 0 | -$200,911 |
| Total Changes | -$248,382 | |||
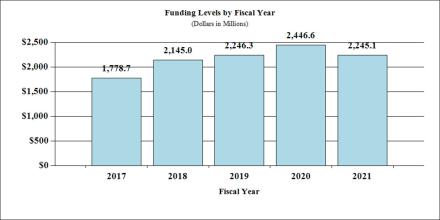
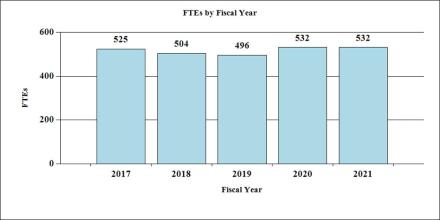
Fiscal Year 2020 Budget Graphs
History of Budget Authority and FTE's:
Distribution of Mechanism:
Change by Selected Mechanism:
Budget Authority by Activity1
(Dollars in Thousands)
| FY 2019 Final | FY 2020 Enacted | FY 2021 President's Budget | FY 2021 +/- FY 2019 | |||||
|---|---|---|---|---|---|---|---|---|
| Extramural Research Detail: | FTEs | Amount | FTEs | Amount | FTEs | Amount | FTEs | Amount |
| Division of Neuroscience | $1,402,821 | $1,534,138 | $1,384,790 | -$149,348 | ||||
| Division of Clinical Research | 116,378 | 126,761 | 115,418 | -11,343 | ||||
| Division of Translational Research | 113,597 | 124,662 | 111,773 | -12,888 | ||||
| Division of Extramural Activities | 100,784 | 110,050 | 100,270 | -9,780 | ||||
| Opiod Research2 | 239,206 | 240,165 | 240,165 | 0 | ||||
| Subtotal, Extramural | $1,972,787 | $2,135,776 | $1,952,416 | -$183,360 | ||||
Intramural Research | 287 | $191,214 | 307 | $219,577 | 307 | $205,665 | 0 | -$13,912 |
| Research Management & Support | 209 | $82,307 | 225 | $91,224 | 225 | $87,029 | 0 | -$4,195 |
| TOTAL | 496 | $2,246,308 | 532 | $2,446,577 | 532 | $2,245,110 | 0 | -$201,467 |
1 Includes F T Es whose payroll obligations are supported by the NIH Common Fund.
2 Total for Opioid/Pain Research including IR and RMS is (in thousands) $240,843 in FY 2019, $$266,321 in FY 2020, and $266,321 in FY 2021.
Authorizing Legislation
| PHS Act/ Other Citation | U.S. Code Citation | 2020 Amount Authorized | FY 2020 Annualized CR | 2021 Amount Authorized | FY 2021 PB | |
|---|---|---|---|---|---|---|
| Research and Investigation | Section 301 | 42§241 | Indefinite | $2,446,577,000 | Indefinite | $2,245,110,000 |
| National Institute of Neurological Disorders and Stroke | Section 401(a) | 42§281 | Indefinite | Indefinite | ||
| Total, Budget Authority | $2,446,577,000 | $2,245,110,000 |
Appropriations History
| Fiscal Year | Budget Estimate to Congress | House Allowance | Senate Allowance | Appropriation |
|---|---|---|---|---|
| 2012 | $1,664,253,000 | $1,664,253,000 | $1,603,741,000 | $1,629,445,000 |
| Rescission | $3,079,651 | |||
| 2013 | $1,624,707,000 | $1,629,631,000 | $1,626,365,349 | |
| Rescission | $3,252,731 | |||
| Sequestration | ($81,632,357) | |||
| 2014 | $1,642,619,000 | $1,631,703,000 | $1,587,982,000 | |
| Rescission | $0 | |||
| 2015 | $1,608,461,000 | $1,605,205,000 | ||
| Rescission | $0 | |||
| 2016 | $1,660,375,000 | $1,656,334,000 | $1,694,758,000 | $1,696,139,000 |
| Rescission | $0 | |||
| 20171 | $1,695,180,000 | $1,751,049,000 | $1,803,306,000 | $1,783,654,000 |
| Rescission | $0 | |||
| 20182 | $1,355,998,000 | $1,853,011,000 | $1,904,666,000 | $2,188,149,000 |
| Rescission | $0 | |||
| 20192 | $1,838,556,000 | $2,228,780,000 | $2,275,580,000 | $2,274,413,000 |
| Rescission | $0 | |||
| 20202 | $2,026,031,000 | $2,385,571,000 | $2,490,494,000 | $2,444,687,000 |
| Recission | $0 | |||
| 20212 | $2,245,110,000 |
1 Budget Estimates to Congress includes mandatory financing.
2 Includes funds derived by transfer from the NIH Innovation Account under the 21st Century Cures Act.
Justification of Budget Request
National Institute of Neurological Disorders and Stroke
Authorizing Legislation: Section 301 and Title IV of the Public Health Service Act, as amended.
Budget Authority (BA):
| FY 2018 Final | FY 2019 Enacted | FY 2019 President's Budget | FY 2020 +/- FY 2019 | |
|---|---|---|---|---|
| BA | $2,246,308,000 | $2,446,577,00 | $2,245,110,000 | -$201,467,000 |
| F T E | 496 | 532 | 532 | 0 |
Director's Overview
The National Institute of Neurological Disorders and Stroke (NINDS) supports research that advances the diagnosis, prevention, and treatment of neurological disorders; that is, diseases of the brain, spinal cord, and nerves of the body. Basic research to understand the brain in health and disease, the wellspring of public and private sector progress, is at the core of this mission.
Chronic pain, dementias, stroke, traumatic brain injury (TBI), epilepsy, Parkinson’s disease, multiple sclerosis, cerebral palsy, and other common neurological disorders affect millions of Americans of all ages. Hundreds of rare diseases collectively add to the enormous impact. Increasing the urgency for progress, brain disorders are becoming more common with the aging of our population, and the high prevalence of chronic pain is a major driver of the opioid crisis.
The multiplicity of neurological diseases, the complexity of the brain, and its sensitivity to disruption present formidable challenges to medical science. To confront this challenge, NINDS supports essential research that the private sector does not–basic studies to understand the brain and its diseases, innovations with long time horizons or high risk of failure, and preventive interventions that pay off in lives saved. Despite the challenges, each year research improves the lives of many people with neurological disorders, building on decades of NINDS and NIH investment. For example: In the 1960s fundamental studies of how nerve cells communicate led to drug therapy with L-DOPA, still the mainstay of Parkinson’s treatment. In the 1970s, NIH investment and advances in physics and engineering launched the revolution in brain imaging, transforming diagnosis and treatment of many brain diseases. In the 1980s, the first enzyme replacement therapy for an inherited metabolic disorder, Gaucher disease, showed the value of a sustained bench-to-bedside research program and provided an early boost to the biotechnology industry, and a pioneering NINDS technology development program moved “neuroprosthetic” devices from science fiction to reality, with the first cochlear implants for the hearing impaired. In the 1990s, research on the immune system and multiple sclerosis led to the first drugs that slow the disease; research on how normal and Parkinson’s disease affected brains control movement guided effective deep brain stimulation (DBS) therapy; and a targeted NINDS development plan, from the laboratory to human trials, developed the first emergency treatment for stroke, with the clot-busting drug tPA, after the private sector had abandoned stroke emergency treatment as too risky for investment.
Since 2000, NINDS research has continued to catalyze private sector therapy development, with several new drugs for multiple sclerosis, epilepsy, insomnia, migraine, and other disorders, and use of DBS to treat epilepsy, dystonia, and other diseases. Advances in genetics, driven by the Human Genome Project, are transforming the outlook for many previously intractable brain diseases. First, better diagnostics eliminated the years’ long diagnostic odysseys for many families confronting rare diseases. More recently, neurogenetics has led to the first wave of drugs and gene therapy, with recent successes for spinal muscular atrophy (SMA), muscular dystrophy, and Batten disease. In a few fortuitous examples, genome sequencing, which is now feasible for individual patients, has led to immediate improvements in care for children with rare forms of dystonia, epilepsy and other disorders. Treatments arising from genetic studies for Huntington’s disease, inherited amyotrophic lateral sclerosis (ALS), Parkinson’s disease, tuberous sclerosis, and several other disorders are now in clinical testing. And, though breakthroughs like gene technologies capture the public’s attention, sustained, incremental improvements in clinical medicine and prevention from dozens of clinical trials and epidemiological studies collectively drove reduction in stroke incidence by 32 percent from 1987 to 2017 for people aged 65 years and older.1
NINDS’s highest priority is investigator-initiated research, because of its long record of driving progress. In addition to traditional NIH grant programs, the Javits Awards to scientists who have exceptional talent, imagination, and achievement, and the highly competitive Research Program Awards, which are available to all but the newest investigators, provide funding for ambitious, creative, or longer-term research projects. Neuroscientists are also remarkably well represented in the NIH-wide Pioneer Awards, Transformative R01, and New Innovator Awards, reflecting the dynamism of the field. For many years, NINDS policies have favored early career investigators. Thus, nearly one third of all NINDS investigators are in their first five years of independent NIH funding, and early-stage investigators receive more NINDS funding than any other cohort. NINDS diversity programs seek to tap the full wealth of the nation’s talent pool to confront the extraordinary challenges that neurological disorders present.
To complement investigator-initiated research, NINDS targets programs to critical public health needs of the present and extraordinary opportunities for the future. The Institute’s intensive engagement in the NIH Helping End Addiction Long-term (HEAL) and the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) initiatives illustrate, respectively, these two types of priorities. The trans-NIH section of the separate Overview volume of the NIH Congressional Justification describes the HEAL and BRAIN programs. Here, the focus is on how, from the perspective of the NINDS mission, BRAIN and HEAL each build on past success and advance the Institute’s mission into the future.
More than 25 million Americans suffer from daily chronic pain.2 The lack of effective, non-addictive treatment for many of these people is a major factor in the opioid crisis. As the largest supporter of pain research, NINDS leads several HEAL initiative programs that are developing non-addictive pain treatments to displace addictive drugs or be effective when opioids are not. HEAL initiatives to identify and validate targets for new pain interventions build on investments in basic pain research and on lessons from how basic research catalyzed private sector development of drugs for SMA, Parkinson’s disease, migraine, and other diseases. HEAL investments in biomarkers leverage NINDS biomarkers programs and recognition that NIH biomarker research stimulates progress. For example, the remarkable new therapies that use clot retrieval devices to directly clear blocked blood vessels in severe strokes when tPA does not restore blood flow rely on a brain imaging biomarker to determine who will benefit. HEAL screening of candidate non-addictive pain drugs follows the model of the NINDS Epilepsy Therapy Screening Program, which has energized the development of anti-seizure drugs by providing to academia and industry a rigorous, independent, standardized evaluation of drug candidates in a series of animal models. HEAL is also adopting the NINDS milestone-gated, preclinical translational research programs, which provide funding, resources, and expertise to basic researchers who identify promising opportunities but lack the resources and expertise for drug development. The HEAL Early Phase Pain Investigational Clinical Network, like pioneering NINDS clinical trials networks, employs innovations such as common institutional review boards that improve the speed and quality of clinical trials.
Rather than responding to a crisis, the BRAIN Initiative is shaping the future, a future in which we understand how dysfunctional brain circuits cause neurological, psychiatric, and substance misuse disorders and how to correct the problems. A convergence of opportunities from many fields of science and engineering launched the initiative, which is, in turn, empowering research across all of neuroscience and brain diseases. More than 40 years ago NINDS pioneered the then science-fiction field of devices that interface with the brain. Now BRAIN is developing ambitious brain computer interfaces that can reconstruct speech from brain signals or restore vision, and DBS methods that self-adjust by monitoring brain activity. Nobel Prize winning NIH research in the 1990s paved the way for optical methods to monitor the activity of neurons. Hundreds of investigators across NIH programs are applying advanced optical methods from the BRAIN Initiative to monitor and modulate brain cells to understand healthy and diseased brain circuits. NIH investment in genomics technologies laid the groundwork for the BRAIN Initiative Cell Census Networks. These methods are now identifying which cell types are affected by dementia, autism, and many other diseases and paving the way toward treatments that precisely target these cells. Beyond neuroscience, the private sector is already investing heavily to improve artificial intelligence (AI), human-computer interfaces, and computer hardware architecture based on understanding of the brain. As remarkable as many of these advances are, history teaches us that the most valuable insights from the BRAIN Initiative, like all basic research, are those that we cannot yet even imagine.
Overall Budget Policy: The FY 2021 President’s Budget Request for NINDS is $2,245.1 million, a decrease of $201.5 million or 8.2 percent compared with the FY 2020 Enacted level. Within this funding level, amounts allocated to the HEAL Initiative are held flat relative to the FY 2020 level, and other funding for opioid and pain research is likewise included at no less than the FY 2020 level.
Program Descriptions and Accomplishments:
Division of Neuroscience (DON)
As the largest part of the NINDS extramural program, DON supports research on the normal brain, spinal cord, and nerves of the body; the mechanisms of neurological disorders; and the early development of diagnostics and treatments. Investigator-initiated research is the foundation for all components of the DON portfolio. Targeted programs, as in the examples below, focus on specific research topics and public health priorities that are not adequately addressed through investigator-initiated programs. DON programs also support research resources, core facilities, and scientific conferences. Program areas include:
- Basic neuroscience research: Gaps in understanding how the nervous system normally develops and functions can form roadblocks to understanding what goes wrong in disease or after injury. Filling those gaps is a critical piece of the NINDS mission and an area unlikely to receive sustained support through the private sector. Because basic research benefits from the freedom to follow scientific opportunity, investigator-initiated, peer-reviewed research is the foundation of the NINDS basic research program. NINDS also promotes basic research through a targeted initiative with set-aside funding. In neuroscience as in other research fields, new research tools drive new discoveries. NINDS supports the development and application of new tools and technologies for neuroscience research, and this is also a major focus of the NIH BRAIN initiative, in which NINDS is a leading partner.
- Epilepsy: Since 2000, NINDS has worked with the scientific community and disease advocates to establish a statement of shared research priorities called the NINDS Benchmarks for Epilepsy Research. The NINDS Centers without Walls program for epilepsy research (CWOW) was designed to address these priorities and has supported Epi4K, a study of genetic causes of epilepsy in over 4,000 people that catalyzed further international collaboration; a center on sudden unexpected death in epilepsy (SUDEP); and the Epilepsy Bioinformatics Study for Antiepileptogenic Therapy, to find strategies to prevent epilepsy after traumatic brain injury. The newest CWOW investigates how specific gene variants cause epilepsy, which will inform precision diagnostics and treatments. An update to the Benchmarks in 2020 will guide future NINDS epilepsy research.
- Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): Researchers do not yet understand the causes of ME/CFS, and diagnostic tests and treatments are not available. Along with the National Institute of Allergy and Infectious Diseases (NIAID) and other NIH Institutes and Centers, NINDS supports ME/CFSnet, a network of collaborative research centers that are applying innovative approaches to study disease mechanisms and engaging the ME/CFS patient and advocacy community in their programs. NINDS will support new funding opportunities for ME/CFS research, and a working group of the National Advisory Neurological Disorders and Stroke (NANDS) Council has outlined additional opportunities to expand research on this complex disease.
- Rare diseases: Many individually rare diseases have neurological components. Research on these conditions often yields broader insights into understanding and treating common diseases that share biological mechanisms. NINDS supports investigator-initiated and targeted research on rare diseases and is a leading collaborator in the NIH Rare Disease Clinical Research Network. NINDS-funded research has enabled innovative new treatments for rare neurological disorders, including the first disease-modifying therapies for spinal muscular atrophy and the first enzyme replacement therapy for direct brain delivery. NINDS will continue to support an initiative for studies that fill gaps in clinical trial readiness for rare neurological and neuromuscular diseases with candidate therapies on the horizon.
- Parkinson’s disease (PD): NINDS support has led to progress in understanding the causes and mechanisms of PD and in developing treatments. Current programs include the Morris K. Udall Centers of Excellence for Parkinson’s Disease Research, supporting collaborative research on the causes of PD; the NINDS Parkinson’s Disease Biomarkers Program (PDBP) to identify biomarkers for PD and Lewy Body dementia that will improve clinical trials and facilitate earlier diagnosis; and the Accelerating Medicines Partnership for Parkinson’s Disease (AMP-PD), a public-private partnership leveraging existing patient cohorts and resources from PDBP and other programs for large-scale analyses designed to find biomarkers and new therapeutic targets. AMP-PD data and analyses will be shared broadly among partners and the biomedical community.
- Traumatic Brain Injury (TBI): NINDS supports research across the range of TBI severity, with studies on injury mechanisms, recovery processes, diagnosis and treatment. Transforming Research and Clinical Knowledge in TBI (TRACK TBI), an observational study of more than 3,000 adults and children with TBI, created a database, analytic tools, and resources to establish precise diagnostic methods, improve outcome assessment, and compare the effectiveness and costs of tests, treatments, and services. The Pediatric Guideline Adherence and Outcomes (PEGASUS) program aims to improve clinical guideline adherence and improve outcomes for pediatric TBI. NINDS and the Department of Defense promote data sharing and research coordination through the Federal Interagency TBI Research (FITBIR) informatics system.
- Other neurological disorders: DON research includes studies on many more neurological disorders and on mechanisms shared across diseases. Recent NINDS-supported advances suggest new paths to treatments for ALS, frontotemporal dementia (FTD), glioblastoma, and spinal cord injury. In 2019, NINDS convened a workshop and sought researcher and public input to identify priorities for spinal cord injury research, including ways to move promising regenerative therapies forward. Building on progress toward protecting the brain after stroke, the Stroke Preclinical Assessment Network will rigorously test potential neuroprotective therapies in rodent models of acute ischemic stroke. Because neuroscience spans scientific disciplines, NINDS will continue to work closely with other NIH Institutes and Centers in areas of complementary interest, including research on pain, Alzheimer’s disease and Alzheimer’s disease-related dementias (AD/ADRD), neuroimmune conditions such as multiple sclerosis and infections of the nervous system, and genetic and neurodevelopmental disorders such as cerebral palsy, autism, muscular dystrophies, and Down syndrome.
Budget Policy: The FY 2021 President’s Budget request for the Division of Neuroscience is $1,384.8 million, a decrease of $149.3 million, or 9.7 percent, from the FY 2020 Enacted level.
FY 2020 Level3: $335.2 million
FY 2021 Level: $305.0 million
Change: -$30.2 million
Congress recognized the public health importance of not only Alzheimer’s disease (AD), but also Alzheimer’s disease-related dementias (ADRD) in the National Alzheimer’s Project Act (NAPA). NINDS leads NIH research on the ADRDs, working closely with the National Institute on Aging (NIA). The ADRDs include vascular contributions to cognitive impairment and dementia (VCID), frontotemporal degeneration (FTD), Lewy body dementia (LBD), and mixed dementias. VCID and Alzheimer’s disease are so intertwined that most elderly people with dementia have a combination of the two, and emerging evidence suggests that interventions to prevent stroke, a brain vascular disorder, may also help in preventing dementia. In 2019, the nationwide NIH Systolic Blood Pressure Intervention Trial Memory and Cognition In Decreased Hypertension (SPRINT-MIND) clinical trial showed that intensive blood pressure control slowed the accumulation of white matter damage in the brain more effectively than standard blood pressure treatment, complementing the trial’s previous finding that the more intensive treatment reduced the chances of developing mild cognitive impairment4.
In March 2019 NINDS led the ADRD Summit, which every third year convenes the scientific and patient advocacy communities to discuss progress, emerging opportunities, and research priorities. In addition to a robust program of investigator-initiated research projects, major new NIH ADRD initiatives focus on identifying which types of stroke present the greatest risk for future dementia, primary care detection of cognitive impairment and dementia including in health disparities populations, the mechanisms that underly LBD, and on brain imaging markers and validation of potential drug targets for ADRD. NINDS and NIA are also collaborating on a major natural history study in FTD that will inform future clinical trials. In FY 2021, new priorities from the 2019 Summit5 include detection and diagnosis of reversible causes of dementia in everyday care, clinical approaches and workforce training for AD/ADRD health disparities, expanded basic science on LBD, the role of a type of pathology called TDP-43 proteinopathy in common dementias, and relations between traumatic brain injury (TBI) and dementias. Finally, NINDS will continue developing biomarkers for ADRD, including validation of VCID biomarkers developed in the MarkVCID consortium.
Division of Translational Research (DTR)
The Division of Translational Research leads extramural NINDS therapy development activities for diseases within the Institute’s mission. DTR has established a suite of milestone-driven research programs and services to support all stages of development for drugs, devices, and biologic therapies, from preclinical studies to first in human clinical trials. Translational research is failure-prone and poses risks for private sector investment. DTR programs are designed to advance promising therapies to a point of readiness sufficient for industry interest, or in some cases, for testing in later stage clinical trials supported by NINDS. In FY 2021, NINDS will continue the following DTR programs.
- The Innovation Grants to Nurture Initial Translational Efforts (IGNITE) program funds early stages of therapy development, including validation of assays to evaluate candidate drugs, demonstrations that proposed therapies have sufficient biological activity to warrant further investment, and development of model systems for early testing. One of IGNITE’s first projects has achieved a primary goal of the program by advancing to the Blueprint Neurotherapeutics Network, to develop a small molecule drug for temporal lobe epilepsy.
- The Blueprint Neurotherapeutics Network (BPN) focuses on the development of small molecule drugs and has supported 25 projects across 8 NIH Institutes and Centers since beginning in 2011. One BPN drug recently entered Phase II clinical trials in patients with early Alzheimer's disease and adults with Fragile X syndrome, a genetic disorder that causes intellectual and developmental disabilities. Other successes include follow-on funding for several projects from licensing and investments, six Investigational New Drug (IND) applications to the FDA, and multiple Phase I clinical trials.
- The Cooperative Research to Enable and Advance Translational Enterprises program (CREATE Bio) supports the development of biologic therapies, including large biological molecules, gene therapies, and cell therapies. Multiple therapies in the program are based on antisense oligonucleotides (ASOs) and small interfering RNAs (siRNAs) designed to modify the expression of specific disease-related genes, reflecting the promise of these precision medicine approaches for neurological disorders. The program is also poised to advance the translation of other novel technologies, such as genome editing, for therapeutic application.
- The Translational Neural Devices program supports the development of therapeutic and diagnostic devices for disorders of the nervous system, from preclinical studies through early stage clinical trials. NINDS also manages device-related programs in the NIH HEAL and BRAIN initiatives and leads a new program through the NIH Blueprint for Neuroscience Research to support training courses for investigators and small business entrepreneurs interested in developing neural devices. NINDS support has contributed to brain stimulation devices for Parkinson’s disease, epilepsy, and other conditions, including the first devices incorporating feedback control based on patients’ ongoing brain activity or symptoms.
- NINDS SBIR/STTR programs support research by small businesses to develop therapies, diagnostics, and research tools relevant to the NINDS mission. NINDS also participates in the NIH Commercialization Readiness Pilot Program for small businesses. An NINDS-supported company recently benefitted from this program to commercialize a new bicycle helmet technology designed to prevent brain injury by protecting against both linear and rotational head accelerations.
- The NINDS Biomarkers program, begun in 2018, supports the development and validation of biomarkers for neurological diseases, which can improve clinical trial design and facilitate treatment decisions. New projects focus on biomarkers of disease progression and treatment response in multiple sclerosis, TBI, neonatal hypoxic-ischemic encephalopathy, and rare genetic conditions. NINDS also leads a program for the discovery and validation of biomarkers for pain indications as part of the NIH HEAL Initiative.
- The Epilepsy Therapy Screening Program (ETSP) screens candidate compounds from academia and industry in standardized models. This longstanding program has helped to advance 10 antiseizure drugs to the market for common and rare forms of epilepsy, including Vimpat (lacosamide) for partial onset seizures, and most recently Epidiolex (cannabidiol) for the treatment of seizures associated with Lennox-Gastaut and Dravet syndromes. The ETSP aims to promote development of new treatments for drug-resistant epilepsy, epilepsy syndromes, and disease prevention and modification.
- The NIH Countermeasures Against Chemical Threats (CounterACT) program, funded from a separate appropriation through the NIH Office of the Director, aims to understand toxicity caused by chemical threat agents and develop interventions to reduce the mortality and morbidity they may cause. CounterACT is part of the NIH Biodefense Program and the Chemical Countermeasures Research Program (CCRP) coordinated by NIAID.
Budget Policy: The FY 2021 President’s Budget request for the Division of Translational Research is $111.8 million, a decrease of $12.9 million, or 10.3 percent, from the FY 2020 Enacted level.
Division of Clinical Research (DCR)
The NINDS Division of Clinical Research supports clinical trials infrastructure and large-scale clinical research, including early and advanced phase clinical trials, comparative effectiveness research, and epidemiological studies. To optimize the efficiency of clinical trials, DCR enforces milestones for progress and provides resources to improve patient access and recruitment. In addition, DCR develops new clinical research initiatives and provides expertise in statistics and clinical trial design to investigators and the Institute. A new Office of Global Health and Health Disparities within DCR will strengthen NINDS investments in research on health disparities and minority, community, and global health.
Over the last several years, DCR-led clinical trials have contributed to advances in the treatment and prevention of neurological disorders including stroke, epilepsy, Parkinson’s disease, multiple sclerosis, and others. DCR also collaborates with other NIH Institutes in areas of shared interest.
In FY 2021, NINDS will continue support for the following DCR programs.
- The Network for Excellence in Neuroscience Clinical Trials (see program portrait)
- StrokeNet provides support and infrastructure for clinical trials on stroke treatment, prevention, and recovery and rehabilitation through 25 regional centers and more than 400 hospitals throughout the U.S. A recent trial completed through StrokeNet found no benefit of tight blood sugar control in stroke patients, a result that addresses a long-standing debate in clinical practice.6 The network’s first rehabilitation trial found that a telehealth program for in-home rehabilitation therapy is effective for improving patient outcomes after stroke.
- Strategies to Innovate EmeRgENcy Care Clinical Trials Network (SIREN) is jointly led by NINDS and the National Heart Lung and Blood Institute (NHLBI) and conducts clinical trials in emergency care for neurologic, cardiac, respiratory, and hematologic conditions. Ongoing trials aim to assess treatments for TBI and for improving neurological outcomes following cardiac arrest with coma. A study in development focuses on treatment for status epilepticus (prolonged seizure).
- The NINDS Common Data Elements (CDE) Program works with research and patient advocacy communities to develop data collection standards for neurological disorders, to facilitate collaboration and data sharing across studies and to enhance data integrity. The program has developed CDEs for 21 neurological disorders and a common set of CDEs for general use across diseases.
- As part of the NIH HEAL Initiative, NINDS leads the Early Phase Pain Investigation Clinical Network (EPPIC-Net), established in 2019 to provide academic and industry investigators with infrastructure and support for early-phase clinical testing of pain therapeutics across populations, with a goal to reduce reliance on opioids by accelerating trials of non-addictive pain therapeutics, including drugs and devices.
Budget Policy: The FY 2021 President’s Budget request for the Division of Clinical Research is $115.4 million, a decrease of $11.3 million, or 8.9 percent, from the FY 2020 Enacted level.
FY 2020 Level: $7.8 million
FY 2021 Level: $7.3 million
Change: -$0.5 million
NINDS created the Network for Excellence in Neuroscience Clinical Trials (NeuroNEXT) in 2011 to expand capabilities for testing promising therapies for neurological disorders, increase the efficiency of clinical trials, and allow researchers to mobilize quickly when opportunities to test new treatments arise. By providing a robust and accessible infrastructure, NeuroNEXT facilitates the rapid development and implementation of study protocols for neurological disorders in adult and pediatric populations. The network is designed to encourage collaborations between academic centers, disease foundations, and industry.
NeuroNEXT consists of a Clinical Coordinating Center at Massachusetts General Hospital, a Data Coordinating Center at the University of Iowa, and 25 clinical sites throughout the country. This infrastructure supports Phase II clinical trials to gather critical information about investigational treatments prior to larger later-stage trials. The network also supports studies to discover and validate measures of disease processes and treatment responses that can be used as biomarkers. NeuroNEXT approaches to improve the efficiency and quality of clinical trials have served as models for other NINDS and NIH networks. For example, NeuroNEXT was among the first at NIH to establish a Central Institutional Review Board, now widely used for expedient review of research protocols and related materials to ensure protection of the rights and welfare of participating human subjects.
To date, nine studies have been conducted through NeuroNEXT, testing biologic and small molecule drug therapies for GNE myopathy, cryptogenic sensory peripheral neuropathy (CSPN), Fragile X Syndrome, glioblastoma, Huntington's disease, ischemic stroke, myasthenia gravis, and multiple sclerosis. Natural history data from a NeuroNEXT study on biomarkers for spinal muscular atrophy (SMA) contributed to the development and approval of the first treatment for this devastating rare disease. In FY 2018, NINDS renewed support for the NeuroNEXT infrastructure for five years, with added emphasis on training opportunities within the network for new clinical investigators. Clinical studies conducted through the network are supported through separate peer-reviewed funding mechanisms.
Division of Extramural Activities
The NINDS Division of Extramural Activities leads NINDS efforts in supporting research training and career development, workforce development initiatives, and activities to enhance rigor and reproducibility in neuroscience research. The main components of DEA include:
- The Office of Training and Workforce Development coordinates NINDS extramural programs for training and career development, including fellowships and mentored awards for individuals and grants for training programs at academic institutions. In addition to participating in NIH-wide programs, NINDS has designed targeted initiatives to meet the unique needs of trainees in neuroscience and neurology at different career stages, such as national programs for child neurologists and neurosurgeons who wish to pursue research and career development awards to help advanced trainees launch independent research projects. To promote excellent mentoring in neuroscience research training, NINDS recently established the Landis Mentor Award, which provides outstanding mentors with support to foster the career advancement of additional trainees.
- The Office of Programs to Enhance Neuroscience Workforce (OPEN) represents NINDS at all levels of NIH in matters pertaining to training and workforce development in the extramural biomedical workforce. OPEN develops and implements individual and institutional funding opportunities while working across the NINDS, Blueprint, and BRAIN scientific portfolios to promote the scientific workforce in the neurosciences. OPEN develops training opportunities and organizes conferences, workshops, symposia, and professional development activities to enhance participation in the neuroscience workforce.
- The Office of Research Quality promotes rigor and transparency in neuroscience research. NINDS efforts in education and awareness about the importance of rigor in research have been instrumental to policies established by NIH and research journals to improve rigor in experimental design and require transparent reporting in research publications. After leading a workshop in 2018 on needs for training in rigorous research practices, the office is compiling educational resources and identifying gaps to facilitate the development of new and improved resources and other training activities.
Intramural Research Program
The NINDS Intramural Research Program (IRP) conducts research and provides research training on the NIH campus in Bethesda, Maryland. The Program spans basic, translational, and clinical research, including neurology and neurosurgery.
- More than 150 laboratories from NINDS and 10 other Institutes conduct neuroscience research at NIH, creating a rich environment for innovative, multidisciplinary studies. Many of these laboratories are housed together in the Porter Neuroscience Research Center, a space designed to encourage collaboration.
- NINDS clinical research benefits from the NIH Clinical Center, a hospital devoted exclusively to clinical investigation. NINDS studies are underway on ME/CFS, Parkinson’s disease, epilepsy, multiple sclerosis, dystonia, brain tumors, neuropathies, and several other rare and more common diseases, including early stage clinical trials using drugs, brain stimulation, and gene therapy. As a participating institute in the NIH Undiagnosed Diseases Program, NINDS investigators help find the causes of puzzling neurological cases referred to the Clinical Center. NINDS also partners with two local emergency departments on studies of acute stroke and TBI.
- The unique resources of the NINDS IRP enable research that bridges basic and clinical neuroscience to understand disease and answer fundamental questions about the nervous system. For example, patients undergoing surgical evaluation for epilepsy at the Clinical Center are important volunteers in NINDS research on memory in the human brain. By recording brain activity from electrode arrays in these patients, NINDS investigators showed that memory retrieval is associated with synchronized, high frequency ripples across brain areas. Another NINDS team uses advanced microscopy methods to probe the inner architecture of neurons and has uncovered surprising roles for proteins involved in two rare neurological disorders.
- In 2019, NINDS welcomed a new Scientific Director for the IRP. Plans under this new leadership include strengthening interactions between basic and clinical research, enhancing diversity and inclusion in the IRP workforce, and modernizing core facilities and instrumentation to enable research probing uncharted scientific questions through access to state-of-the-art research technologies.
Budget Policy: The FY 2021 President’s Budget request for the NINDS Intramural Research Program is $205.7 million, a decrease of $13.9 million, or 6.3 percent, from the FY 2020 Enacted level.
Research Management and Support (RMS)
RMS activities provide administrative, budgetary, logistical, and scientific support in the review, award, and monitoring of research grants, training awards, and research and development contracts. RMS functions also encompass strategic planning, coordination, and evaluation of NINDS programs, regulatory compliance, international coordination, and liaison with other Federal agencies, Congress, and the public, as well as NINDS activities to communicate research advances and disseminate information about neurological disorders to the public.
Budget Policy: The FY 2021 President’s Budget request for NINDS Research Management and Support is $87.0 million, a decrease of $4.2 million, or 4.6 percent, from the FY 2020 Enacted level.
HEAL Initiative
NINDS is a leading partner in the NIH HEAL Initiative, providing leadership and support for research to develop new non-addictive pain therapies. For more information, see the trans-NIH section on the NIH HEAL Initiative in the Overview volume of the NIH Congressional Justification.
__________________________________________
1 JAMA Neurology 2019 Sept 30; doi: 10.1001/jamaneurol.2019.3258
2 Journal of Pain 2015;16(8):769-780
3 Estimated dollar amounts includes NIA funding.
4 JAMA. 2019 Feb 12;321(6):553-561. doi: 10.1001/jama.2018.21442. PubMed PMID: 30688979
6 JAMA. 2019 Jul 23;322(4):326-335.doi: 10.1001/jama.2019.9346. PubMed PMID: 31334795
Budget Authority by Object Class 1
(Dollars in Thousands)
| FY 2020 Enacted | FY 2021 President's Budget | FY 2021 +/- FY 2020 | |
|---|---|---|---|
| Total compensable workyears: | |||
| Full-time equivalent | 532 | 532 | 0 |
| Full-time equivalent of overtime and holiday hours | 0 | 0 | 0 |
| Average ES salary | $192 | $192 | $0 |
| Average GM/GS grade | 12.6 | 12.6 | 0.0 |
| Average GM/GS salary | $120 | $122 | $2 |
| Average salary, grade established by act of July 1, 1944 (42 U.S.C. 207) | $109 | $113 | $3 |
| Average salary of ungraded positions (in whole dollars) | $141 | $146 | $5 |
OBJECT CLASSES | FY 2020 Enacted | FY 2021 President's Budget | FY 2021+/- FY 2020 |
|---|---|---|---|
| Personnel Compensation: | |||
| 11.1 Full-time permanent | $37,595 | $38,027 | $432 |
| 11.3 Other than full-time permanent | 28,224 | 28,549 | 325 |
| 11.5 Other personnel compensation | 1,835 | 1,856 | 21 |
| 11.7 Military personnel | 521 | 534 | 14 |
| 11.8 Special Personnel Services Payments | 9,998 | 10,113 | 115 |
| 11.9 Subtotal Personnel Compensation | $78,173 | $79,080 | $907 |
| 12.1 Civilian Personnel benefits | 23,007 | 23,904 | 897 |
| 12.2 Military Personnel Benefits | 324 | 333 | 9 |
| 13.0 Benefits to Former Personnel | 0 | 0 | 0 |
| Subtotal, Pay Costs | $101,504 | $103,317 | $1,813 |
| 21.0 Travel and Transportation of Persons | 4,304 | 4,364 | 60 |
| 22.0 Transportation of Things | 216 | 220 | 4 |
| 23.2 Rental Payments to Others | 162 | 165 | 3 |
| 23.3 Communications, Utilities and Miscellaneous Charges | 639 | 652 | 13 |
| 24.0 Printing & Reproduction | 5 | 5 | 0 |
| 25.1 Consulting Services | 813 | 830 | 16 |
| 25.2 Other Services | 65,668 | 61,334 | -4,334 |
| 25.3 Purchase of goods and services from government accounts | 207,652 | 195,864 | -11,788 |
| 25.4 Operation & Maintenance of Facilities | 2,805 | 2,806 | 2 |
| 25.5 R&D Contracts | 50,110 | 43,094 | -7,017 |
| 25.6 Medical Care | 301 | 312 | 12 |
| 25.7 Operation & Maintenance of Equipment | 3,825 | 3,901 | 76 |
| 25.8 Subsistence & Support of Persons | 55 | 56 | 1 |
| 25.0 Subtotal Other Contractual Services | $331,229 | $308,197 | -$23,032 |
| 26.0 Supplies & Materials | $11,354 | $10,245 | -$1,109 |
| 31.0 Equipment | 10,636 | 3,415 | -7,221 |
| 32.0 Land and Structures | 0 | 0 | 0 |
| 33.0 Investments & Loans | 0 | 0 | 0 |
| 41.0 Grants, Subsidies & Contributions | 1,986,521 | 1,814,523 | -171,999 |
| 42.0 Insurance Claims & Indemnities | 0 | 0 | 0 |
| 43.0 Interest & Dividends | 2 | 2 | 0 |
| 44.0 Refunds | 0 | 0 | 0 |
| Subtotal Non-Pay Costs | $2,345,073 | $2,141,793 | -$203,280 |
| Total Budget Authority by Object Class | $2,446,577 | $2,245,110 | -$201,467 |
1Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
Salaries and Expenses
(Dollars in Thousands)
| OBJECT CLASSES | FY 2020 Enacted | FY 2021 President's Budget | FY 2021 +/- FY 2020 |
|---|---|---|---|
| Personnel Compensation: | |||
| Other Contractual Services: | |||
| Full-Time Permanent (11.1) | $37,595 | $38,027 | $432 |
| Other Than Full-Time Permanent (11.3) | 28,224 | 28,549 | 325 |
| Other Personnel Compensation (11.5) | 1,835 | 1,856 | 21 |
| Military Personnel (11.7) | 521 | 534 | 14 |
| Special Personnel Services Payments (11.8) | 9,998 | 10,113 | 115 |
| Subtotal Personnel Compensation (11.9) | $78,173 | $79,080 | $907 |
| Civilian Personnel Benefits (12.1) | $23,007 | $23,904 | $897 |
| Military Personnel Benefits (12.2) | 324 | 333 | 9 |
| Benefits to Former Personnel (13.0) | 0 | 0 | 0 |
| Subtotal Pay Costs | $101,504 | $103,317 | $1,813 |
| Travel & Transportation of Persons (21.0) | $4,304 | $4,364 | $60 |
| Transportation of Things (22.0) | 216 | 220 | 4 |
| Rental Payments to Others (23.2) | 162 | 165 | 3 |
| Communications, Utilities and Misc. Charges (23.3) | 639 | 652 | 13 |
| Printing and Reproduction (24.0) | 5 | 5 | 0 |
| Consultant Services (25.1) | 813 | 830 | 16 |
| Other Services (25.2) | 65,018 | 60,671 | -4,347 |
| Purchases from government accounts (25.3) | 144,282 | 135,887 | -8,396 |
| Operation and Maintenance of Facilities (25.4) | 2,805 | 2,806 | 2 |
| Operation and Maintenance of Equipment (25.7) | 3,825 | 3,901 | 76 |
| Subsistence and Support of Persons (25.8) | 55 | 56 | 1 |
| Subtotal Other Contractual Services | $216,798 | $204,151 | -$12,647 |
| Supplies and Materials (26.0) | $11,354 | $10,245 | -$1,109 |
| Subtotal Non-Pay Costs | $233,478 | $219,802 | -$13,676 |
| Total Administrative Costs | $334,983 | $323,119 | -$11,863 |
Details of Full-Time Equivalent Employment (FTE's)
| OFFICE/DIVISION | FY 2019 Final | FY 2020 Enacted | FY 2021 President's Budget | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Civilian | Military | Total | Civilian | Military | Total | Civilian | Military | Total | |
| Office of the Director | |||||||||
| Division of Clinical Research | |||||||||
| Division of Translational Research | |||||||||
| Division of Intramural Research | |||||||||
| Division of Extramural Activities | |||||||||
| Division of Neuroscience | |||||||||
| Includes FTEs whose payroll obligations are supported by the NIH Common Fund. | |||||||||
| Direct: | 67 | - | 67 | 70 | - | 70 | 70 | - | 70 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 67 | - | 67 | 70 | 70 | 70 | - | 70 | |
| Direct: | 14 | - | 14 | 17 | - | 17 | 17 | - | 17 |
| Reimbursable: | 1 | - | 1 | 1 | - | 1 | 1 | - | 1 |
| Total: | 15 | - | 15 | 18 | - | 18 | 18 | - | 18 |
| Direct: | 22 | - | 22 | 25 | - | 25 | 25 | - | 25 |
| Reimbursable: | 2 | - | 2 | 2 | - | 2 | 2 | - | 2 |
| Total: | 24 | - | 24 | 27 | - | 27 | 27 | - | 27 |
| Direct: | 271 | 3 | 274 | 290 | 4 | 294 | 290 | 4 | 294 |
| Reimbursable: | 13 | - | 13 | 13 | - | 13 | 13 | - | 13 |
| Total: | 284 | 3 | 287 | 303 | 4 | 307 | 303 | 4 | 307 |
| Direct: | 57 | - | 57 | 60 | - | 60 | 60 | - | 60 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 57 | - | 57 | 60 | - | 60 | 60 | - | 60 |
| Direct: | 42 | - | 42 | 46 | - | 46 | 46 | - | 46 |
| Reimbursable: | 4 | - | 4 | 4 | - | 4 | 4 | - | 4 |
| Total: | 46 | - | 46 | 50 | - | 50 | 50 | - | 50 |
| Total | 493 | 3 | 496 | 528 | 4 | 532 | 528 | 4 | 532 |
| FTEs supported by funds from Cooperative Research and Development Agreements | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
FTEs supported by funds from Cooperative Research and Development Agreements.
| FISCAL YEAR | Average GS Grade |
|---|---|
| 2017 | 12.3 |
| 2018 | 12.5 |
| 2019 | 12.6 |
| 2020 | 12.6 |
| 2021 | 12.6 |
Detail of Positions1
(Dollars in Thousands)
| GRADE | FY 2019 Final | FY 2020 Enacted | FY 2021 President's Budget |
|---|---|---|---|
| Total, ES Positions | 1 | 1 | 1 |
| Total, ES Salary | 192,254 | 192,254 | 192,254 |
| GM/GS-15 | 61 | 65 | 65 |
| GM/GS-14 | 58 | 63 | 63 |
| GM/GS-13 | 105 | 110 | 110 |
| GS-12 | 53 | 54 | 54 |
| GS-11 | 16 | 15 | 15 |
| GS-10 | 2 | 2 | 2 |
| GS-9 | 12 | 15 | 15 |
| GS-8 | 7 | 8 | 8 |
| GS-7 | 8 | 1 | 1 |
| GS-6 | 1 | 1 | 1 |
| GS-5 | 0 | 0 | 0 |
| GS-4 | 3 | 3 | 3 |
| GS-3 | 0 | 0 | 0 |
| GS-2 | 3 | 0 | 0 |
| GS-1 | 1 | 1 | 1 |
| Subtotal | 330 | 338 | 338 |
| Grades established by Act of July 1, 1944 (42 U.S.C. 207): | |||
| Assistant Surgeon General | 0 | 0 | 0 |
| Director Grade | 1 | 1 | 1 |
| Senior Grade | 0 | 0 | 0 |
| Full Grade | 2 | 3 | 3 |
| Senior Assistant Grade | 0 | 0 | 0 |
| Assistant Grade | 0 | 0 | 0 |
| Subtotal | 3 | 4 | 4 |
| Ungraded | 194 | 210 | 210 |
| Total permanent positions | 327 | 336 | 336 |
| Total positions, end of year | 528 | 553 | 553 |
| Total full-time equivalent (F T E) employment, end of year | 496 | 532 | 532 |
| Average ES salary | 192,254 | 192,254 | 192,254 |
| Average GM/GS grade | 12.6 | 12.6 | 12.6 |
| Average GM/GS salary | 117,915 | 119,719 | 120,550 |
1 Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
