NIH BRAIN Initiative Cell Census Network (BICCN): The first complete cell census and atlas of a mammalian brain and a quest toward defining the human brain
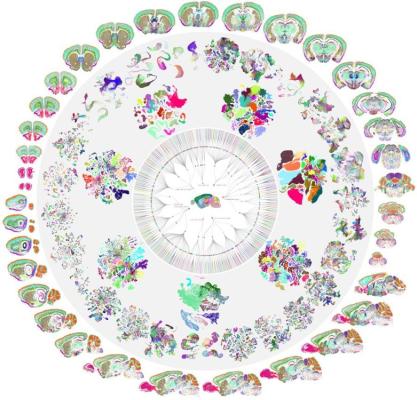
The BRAIN Initiative - Cell Census Network (BICCN) is a consortium of centers in the United States and Europe that work cooperatively to characterize the vast multitude of brain cell types and functions. After five years of extensive work funded by The Brain Initiative®, the BICCN produced an atlas, published in a collection of 10 papers in the journal Nature, that describes the type, location, and identity of more than 32 million cells in the mouse brain. The atlas also delineates the neurotransmitters and neuropeptides used by different cells and the relationship among cell types. This information creates a detailed blueprint for how chemical signals are initiated and transmitted in different parts of the brain. In a parallel effort, published in 21 papers across Science, Science Advances, and Science Translational Medicine, the BICCN compared over 3 million cells across human, nonhuman primate, and rodent brains across developmental periods. This triumph of team science has helped reveal what makes us uniquely human and sets the stage for the BRAIN Initiative Cell Atlas Network which hopes to generate a complete reference atlas of cells in the human brain across development.
Image: A circular "mandala" type diagram that starts with a whole mouse brain in the center and expands out to multi-colored cell-type clusters. The circle is bordered by multicolored coronal and sagittal flat brain sections where the colors represent different types of RNA.
Articles:
BICCN: The first complete cell census and atlas of a mammalian brain
Science: A quest into the human brain
NIH Press Release(s):
Scientists Unveil Complete Cell Map of a Whole Mammalian Brain
Epidural stimulation of the cervical spinal cord for post-stroke upper-limb paresis
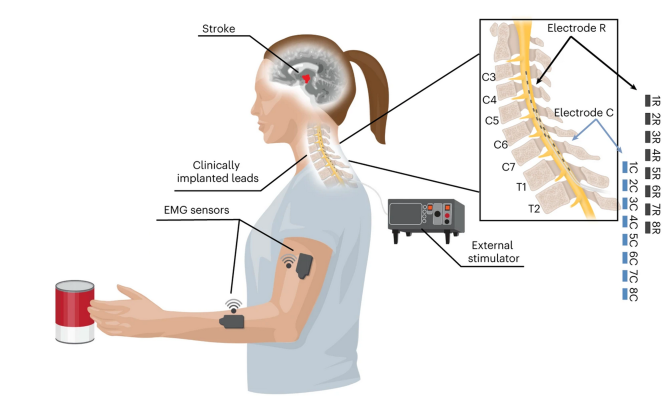
Globally, one in four people over the age of 25 will suffer from a stroke. Nearly three-quarters of these individuals exhibit lasting deficits in motor control of their arm and hand. In this study, funded by the National Institutes of Health’s Brain Research Through Advancing Innovative Neurotechnologies® (BRAIN) Initiative and published in Nature Medicine, spinal cord stimulation of the cervical sensory nerve roots was able to restore motor function in two stroke patients without any adverse issues. Researchers provided continuous stimulation through two linear implants, which amplified the connection between patients’ cortex and cervical spinal circuits. This improved strength, range of motion, function of the arm and hand, and allowed patients to perform complex tasks, like eating with utensils. By the end of the study, these benefits persisted even when stimulation was turned off. While this study was limited in scope, it demonstrates an exciting avenue for future therapeutic device development.
Image: A cartoon representation of a the research setup, showing an individual performing a reaching task. The image depicts the site of stroke in the brain, wireless EMG sensors on the arm and forearm, a depiction of an external stimulator, and an indicator of where leads were implanted. To the upper right is an enlarged image depicting the anatomy of the cervical and thoracic spinal column that depicts the area where each electrode made contact.
Articles:
Powell M et al. Epidural stimulation of the cervical spinal cord for post-stroke upper-limb paresis. Nature Medicine. February 20th, 2023
NIH Press Release: Study finds spinal cord stimulation may restore arm and hand mobility after stroke
First-in-human prediction of chronic pain state using intracranial neural biomarkers
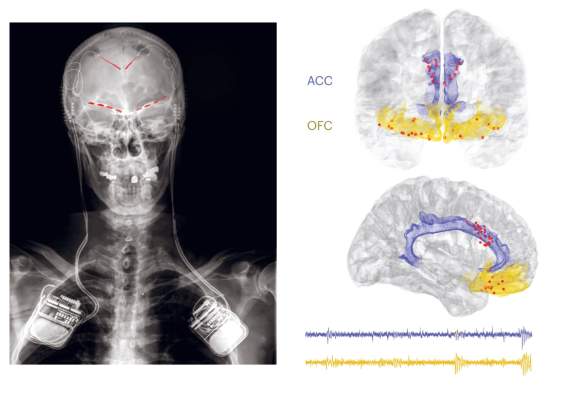
Chronic neuropathic pain syndromes such as post-stroke and phantom limb pain are often resistant to treatment. This type of pain has been measured through subjective self-report, which limits how well data can be compared across individuals. In this study, funded by The BRAIN Initiative® and the NIH HEAL Initiative® and published in Nature Neuroscience, researchers implanted electrodes in four people with chronic neuropathic pain to record neural activity in two brain regions thought to integrate sensory, emotional, and cognitive dimensions of pain: the anterior cingulate cortex (ACC) and the orbitofrontal cortex (OFC). By allowing patients to initiate recordings multiple times a day over months and using machine learning to interpret the data, researchers were able to identify neural signals that worked as objective markers of pain. They were also able to differentiate these signals from those caused by acute pain in the same patients. These tools are a leap forward in developing novel methods for tracking and treating chronic pain.
Image: Left: An x-ray showing where electrode leads are placed in the brain and where the recording device was implanted near the shoulders. Right, top: A representation of a frontal view of the brain where the ACC is colored in blue, the OFC is colored in yellow, and red dots show where electrode leads were placed. Right, bottom: A side view of the brain with the same color-scheme. Below it are lines that are raw recorded data from the implanted electrodes. The ACC line is blue and the OFC line is yellow.
Articles:
Shirvalkar P et al. First-in-human prediction of chronic pain state using intracranial neural biomarkers. Nature Neuroscience. May 22nd, 2023.
NIH Press Release(s):
Brain signatures for chronic pain identified in a small group of individuals
Brain activity predicts chronic pain
Deep Phenotyping of Neurologic Post-Acute Sequelae of SARS-CoV-2 Infection
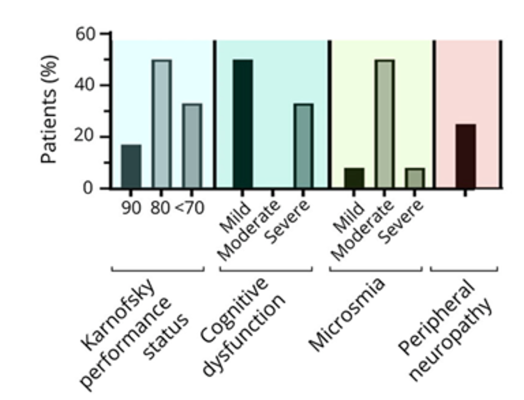
Dr. Mina and colleagues aimed to comprehensively characterize the poorly understood neuro-PASC features linked to SARS-CoV-2 infection. Participants were evaluated at a median of 9-months post-COVID-19 and compared with healthy volunteers. The main findings include the persistence of cognitive difficulties and fatigue, highly disabling conditions, and smell dysfunction. Brain MRI scans were mostly normal. Cerebrospinal fluid analysis identified intrathecal oligoclonal bands in three cases. Participants showed abnormalities in both CD4+ and CD8+ T cells, alongside increased frequencies of antibody-secreting B cells and cells expressing immune checkpoint molecules. Taken together, the findings add to growing evidence that widespread immunological and autonomic nervous system changes may contribute to Long COVID. The results may help researchers better characterize the condition and explore possible therapeutic strategies, such as immunotherapy.
Image: Symptoms of neuro-PASC; bars on the right represent the frequency of symptoms in the cohort (%), whereas the bars on the left represent the time of onset after SARS-CoV-2 infection (mean ± SD).
Articles:
Mina, Y., Enose-Akahata, Y., Hammoud, D. A., Videckis, A. J., Narpala, S. R., O'Connell, S. E., Carroll, R., Lin, B. C., McMahan, C. C., Nair, G., Reoma, L. B., McDermott, A. B., Walitt, B., Jacobson, S., Goldstein, D. S., Smith, B. R., & Nath, A. (2023). Deep Phenotyping of Neurologic Postacute Sequelae of SARS-CoV-2 Infection. Neurology(R) Neuroimmunology & Neuroinflammation, 10(4), e200097. PubMed: Observational Study
NIH Press Release: NIH study identifies features of Long COVID neurological symptoms
Safety and clinical activity of autologous RNA chimeric antigen receptor T-cell therapy in myasthenia gravis (MG-001): a prospective, multicenter, open-label, non-randomized phase 1b/2a study
Myasthenia gravis (MG) is a chronic autoimmune disorder in which antibodies destroy the communication between nerves and muscle, resulting in weakness of the skeletal muscles. To address the limitations associated with the use of chimeric antigen receptor (CAR) T cells in individuals with autoimmune disease, Dr. Granit and colleagues engineered CAR-T cells, Descartes- 08, with RNA (rCAR-T) instead of the conventional DNA method, allowing for a short course of treatment that is given multiple times instead of the single-dose regimen normally used in DNA programmed CAR-T therapy. This novel approach was tested in individuals with MG: Descartes-08 appeared to be safe and well tolerated, showing clinically meaningful decreases in MG severity, indicating Descartes-08 as a useful, as-needed treatment. Though early data on the treatment’s effectiveness are promising, additional clinical studies are needed: Descartes-08 therapy is now being tested in a larger, clinical trial to determine its ability to reduce myasthenia gravis symptoms.
Image: Colorized scanning electron micrograph of a T lymphocyte (also known as a T cell) (blue).
Image source: NIAID Potential treatment for rare autoimmune disorder adapted from CAR-T therapy
Articles:
Granit, V., Benatar, M., Kurtoglu, M., Miljković, M. D., Chahin, N., Sahagian, G., Feinberg, M. H., Slansky, A., Vu, T., Jewell, C. M., Singer, M. S., Kalayoglu, M. V., Howard, J. F., Jr, Mozaffar, T., & MG-001 Study Team (2023). Safety and clinical activity of autologous RNA chimeric antigen receptor T-cell therapy in myasthenia gravis (MG-001): a prospective, multicentre, open-label, non-randomised phase 1b/2a study. The Lancet. Neurology, 22(7), 578–590. PubMed: Clinical Trial
NIH Press Release: Potential treatment for rare autoimmune disorder adapted from CAR-T therapy
Identification of genetic risk loci and causal insights associated with Parkinson's disease in African and African admixed populations: a genome-wide association study
Using a comprehensive Genome-Wide Association Study, Dr. Rizig and colleagues identified a novel genetic risk factor in GBA1 in people of African and African admixed ancestry, which has not been seen in European populations. The study revealed that that the GBA1 rs3115534-G variant, present in 39% of the sample, was linked to reduced glucocerebrosidase activity, a factor associated with the identified risk of Parkinson's disease. Moreover, the GBA1 rs3115534-G variant served as a modifier for age at onset of the disease. These results suggest that the GBA1 rs3115534-G variant could be a major mechanistic basis of Parkinson’s disease in African populations. The findings underscore the importance of inclusive representation of ancestrally diverse populations in genetic research.
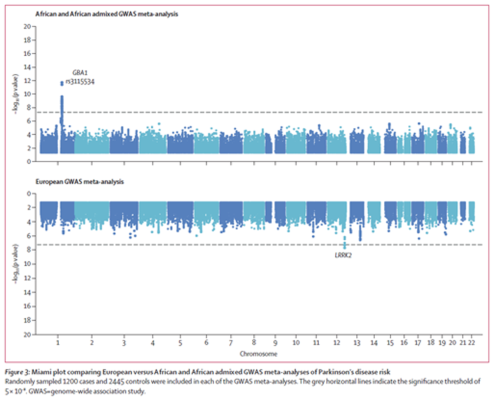
Image: Miami plot comparing European versus African and African admixed GWAS meta-analyses of Parkinson’s disease risk. Randomly sampled 1200 cases and 2445 controls were included in each of the GWAS meta-analyses. The grey horizontal lines indicate the significance threshold of 5 × 10–⁸.
Articles:
Rizig, M., Bandres-Ciga, S., Makarious, M. B., Ojo, O. O., Crea, P. W., Abiodun, O. V., Levine, K. S., Abubakar, S. A., Achoru, C. O., Vitale, D., Adeniji, O. A., Agabi, O. P., Koretsky, M. J., Agulanna, U., Hall, D. A., Akinyemi, R. O., Xie, T., Ali, M. W., Shamim, E. A., Ani-Osheku, I., … Global Parkinson's Genetics Program (2023). Identification of genetic risk loci and causal insights associated with Parkinson's disease in African and African admixed populations: a genome-wide association study. The Lancet. Neurology, 22(11), 1015–1025.
PubMed: Meta Analysis
NIH Press Release: Parkinson’s disease gene variant found in study of some people of African ancestry
External Press Release:
Why is diversity in Parkinson’s research so important?
New gene variant linked to Parkinson's disease found in people of African ancestry
Research Identifies Novel Genetic Risk Factor of Parkinson Disease in African Populations
NINDS Health Equity Strategic Planning Process Overview, High-Level Recommendations, and Guide
The National Advisory Neurological Disorders and Stroke Council presented a comprehensive summary, outlining the direction for advancing the NINDS' health equity strategic planning initiative to eliminate disparities in neurologic conditions. Ten articles were identified to provide readers with context, evidence, and scientific reasoning for each of the 18 recommendations. The recommendations emphasize the need for increased research funding across all neurologic disease areas to identify and validate approaches to eliminate disparities. Strategies involve community engagement, training programs, and career development initiatives to promote diversity and inclusion in health equity research. The recommendations also emphasize the creation of tools, databases, and surveillance systems to enhance early detection, prevention, and evidence-based care for neurologic diseases in vulnerable populations.
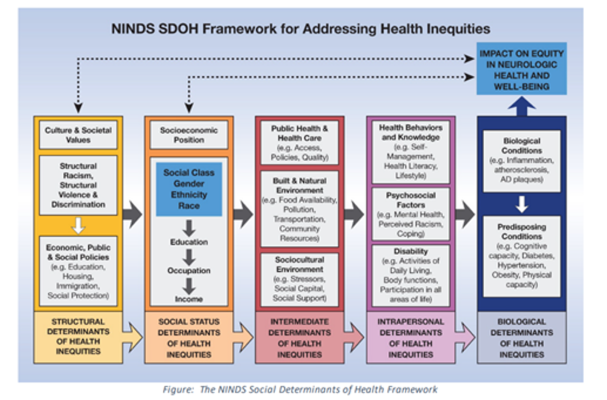
Image: Determinants of Inequities in Neurological Disease, Health, & Well-being: The NINDS Social Determinants of Health Framework for Addressing Health Inequities
Image Source: Griffith, D. M., Towfighi, A., Manson, S. M., Littlejohn, E. L., & Skolarus, L. E. (2023). Determinants of Inequities in Neurologic Disease, Health, and Well-being: The NINDS Social Determinants of Health Framework. Neurology, 101(7 Suppl 1), S75–S81.
Articles:
Johnston, K. C., & Trevathan, E. (2023). NINDS Health Equity Strategic Planning Process Overview, High-Level Recommendations, and Guide. Neurology, 101(7 Suppl 1), S1–S8.
NIH Press Release: NINDS announces neurological health equity strategic plan
External Press Release: Neurological Health Equity: Research for All
Monocyte-derived IL-6 programs microglia to rebuild damaged brain vasculature
Cerebrovascular injury (CVI) can occur after infections, injury, stroke, neurodegeneration and autoimmune disease, resulting in damage to the surrounding brain tissues. In a study published in Nature Immunology, Dr. Bo-Ran Choi, a postdoctoral fellow in the lab of Dr. Dorian McGavern, found that CVI induces the generation of repair-associated microglia (RAMs) and that the number of RAMs is correlated with CVI severity. They demonstrated that infiltrating monocytes (a type of white blood cell) and interlukin-6 (a protein important for immune system signaling) are necessary for RAMs to be “programmed” to repair tissue after CVI. These data provide a molecular and cellular basis for how monocytes instruct microglia to repair brain vasculature and promote recovery after injury and suggest that future treatments aimed at increasing RAM numbers could help CVI patients.
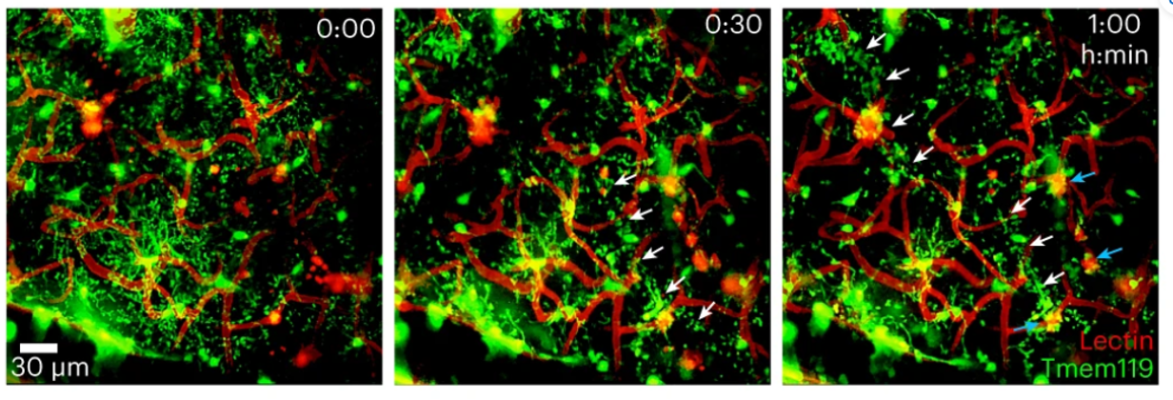
Image: Figure 1B. Time lapse microscope images show microglia (Tmem119; green) interacting with blood vessels (Lectin; red) 30 minutes and 1 hour after CVI in vivo. White arrows indicate microglia extending processes toward a damaged vessel. Cyan arrows indicate microglia interactions with extravasated, lectin-derived material.
Article: Choi, B. R., Johnson, K. R., Maric, D., & McGavern, D. B. (2023). Monocyte-derived IL-6 programs microglia to rebuild damaged brain vasculature. Nature immunology, 24(7), 1110–1123. PubMed
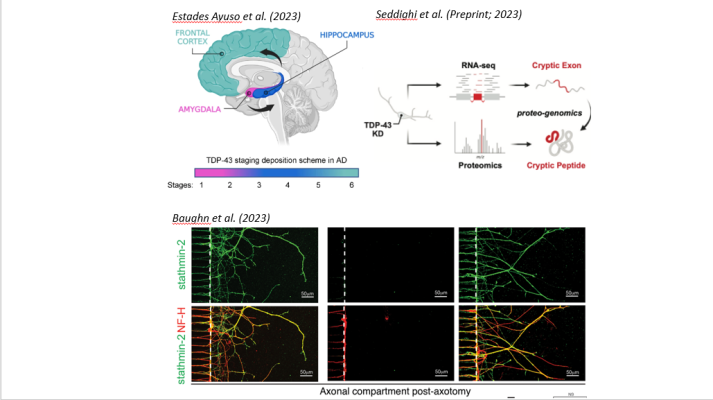
Three papers shed light on mechanisms underlying TDP-43 proteinopathy
Abnormalities in the transcriptional repressor TAR DNA-binding protein-43 (TDP-43) are linked to amyotrophic lateral sclerosis (ALS), Alzheimer’s Disease (AD), frontotemporal dementia (FTD) and limbic-predominant age-related TDP-43 encephalopathy (LATE). This year, three seminal papers shed light on mechanisms of TDP-43 proteinopathy. Three seminal new papers investigate how TDP-43 dysfunction leads to misspliced “cryptic” RNAs. Estades Ayuso and colleagues show that the distribution of cryptic RNA accumulation in the brains of individuals with AD mimicked that of phosphorylated TDP-43, and that cryptic RNA accumulation could efficiently distinguish AD patients from controls. In a new preprint, Seddighi and colleagues show that TDP-43 depletion in iPSC-derived neurons leads to de novo “cryptic” peptides, altering protein-protein interactions. Many of these de novo peptides were also present in the cerebrospinal fluid of patients with ALS. Finally, Baughn and colleagues provide mechanistic insight into the function of TDP-43 and identify two methods to restore function of the protein stathmin-2 in motonuerons affected by TDP-43 pathology in vitro, laying the groundwork for the development of potential therapeutic approaches.
Images:
TOP LEFT: Estades Ayuso et al. (2023) Figure 1A. TDP-43 staging scheme in Alzheimer’s disease.
TOP RIGHT: Seddighi et al. (Preprint; 2023) Figure 2A.
Illustration of how RNA sequencing and proteomic methods were used for identifying “cryptic” exons and peptides in TDP-43 deficient neurons.
BOTTOM: Baughn et al. (2023) Figure 4B. Microscope imaging showing in vitro axonal regeneration in neurons with TDP-43 pathology before rescue of the gene encoding stathmin-2. Left: Controls; Middle: TDP-43 pathology, Right: TDP pathology after treatment. Axons are labeled in red, stathmin-2 is labeled in green; yellow indicates areas of overlap.
Estades Ayuso, V., Pickles, S., Todd, T., Yue, M., Jansen-West, K., Song, Y., González Bejarano, J., Rawlinson, B., DeTure, M., Graff-Radford, N. R., Boeve, B. F., Knopman, D. S., Petersen, R. C., Dickson, D. W., Josephs, K. A., Petrucelli, L., & Prudencio, M. (2023). TDP-43-regulated cryptic RNAs accumulate in Alzheimer's disease brains. Molecular neurodegeneration, 18(1), 57. PubMed
Seddighi, S., Qi, Y. A., Brown, A. L., Wilkins, O. G., Bereda, C., Belair, C., Zhang, Y., Prudencio, M., Keuss, M. J., Khandeshi, A., Pickles, S., Hill, S. E., Hawrot, J., Ramos, D. M., Yuan, H., Roberts, J., Kelmer Sacramento, E., Shah, S. I., Nalls, M. A., Colon-Mercado, J., … Ward, M. E. (2023). Mis-spliced transcripts generate de novo proteins in TDP-43-related ALS/FTD. bioRxiv : the preprint server for biology, 2023.01.23.525149. PubMed
Baughn, M. W., Melamed, Z., López-Erauskin, J., Beccari, M. S., Ling, K., Zuberi, A., Presa, M., Gonzalo-Gil, E., Maimon, R., Vazquez-Sanchez, S., Chaturvedi, S., Bravo-Hernández, M., Taupin, V., Moore, S., Artates, J. W., Acks, E., Ndayambaje, I. S., Agra de Almeida Quadros, A. R., Jafar-Nejad, P., Rigo, F., … Cleveland, D. W. (2023). Mechanism of STMN2 cryptic splice-polyadenylation and its correction for TDP-43 proteinopathies. Science (New York, N.Y.), 379(6637), 1140–1149. PubMed
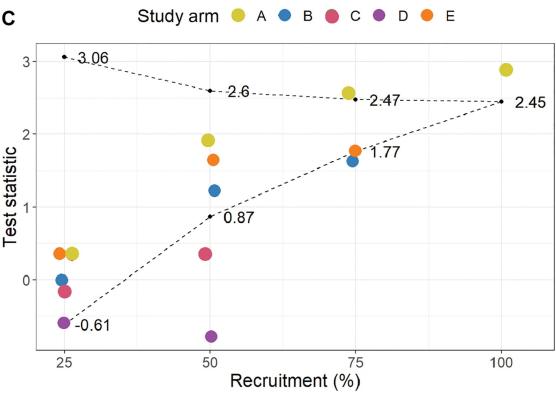
A multi-laboratory preclinical trial in rodents to assess treatment candidates for acute ischemic stroke
Researchers have identified uric acid as a potential therapy to enhance recovery from acute ischemic stroke using a new method for conducting preclinical animal research. In the study, researchers from the National Institutes of Health’s Stroke Preclinical Assessment Network (SPAN) rigorously tested the effectiveness of six novel therapies in reducing ischemic brain injury in rodents using strategies normally reserved for clinical studies in humans.
In addition to incorporating increased scientific rigor into preclinical testing, researchers used animal models that resembled typical stroke patients. The study included young mice and rats, aging mice, mice with diet-induced obesity or hyperglycemia, and rats with spontaneous hypertension, with equal numbers of males and females. (Visit A multi-laboratory preclinical trial in rodents to assess treatment candidates for acute ischemic stroke)
Image: Left: Figure 3A. Schematic showing the behavioral corner test used to assess whether rodents prefer to turn left or right when approaching a corner. Animals will preferentially turn to the side that is ipsilateral to the stroke location in their brain. Of the treatments tested, only uric acid (A) was above the efficacy boundary (top dotted line). Study arms: A, uric acid; B, tocilizumab; C, fingolimod; D, veliparib; E, fasudil.
Lyden, P. D., Diniz, M. A., Bosetti, F., Lamb, J., Nagarkatti, K. A., Rogatko, A., Kim, S., Cabeen, R. P., Koenig, J. I., Akhter, K., Arbab, A. S., Avery, B. D., Beatty, H. E., Bibic, A., Cao, S., Simoes Braga Boisserand, L., Chamorro, A., Chauhan, A., Diaz-Perez, S., Dhandapani, K., … Sansing, L. H. (2023). A multi-laboratory preclinical trial in rodents to assess treatment candidates for acute ischemic stroke. Science translational medicine, 15(714), eadg8656.
Press release: Researchers develop new method to identify potential stroke therapies
As we head into the new year at NINDS, it’s time again to reflect on all that has come and gone in the past twelve months. Even as we face complex challenges in neuroscience research and the diseases and conditions we hope to improve, I am proud of the progress we and our many partners have achieved. In 2023, NINDS supported and conducted research leading to new advances in basic, translational, and clinical neuroscience. While I cannot include all of them here, I have selected a few that you can view above, and I invite you to read about other highlights from the year below. We are grateful for the breadth of perspectives and expertise that made these and other accomplishments possible.
BRAIN celebrates milestone achievements
2023 marked 10 years since the White House first announced the Brain Research Through Advancing Innovative Neurotechnologies® Initiative, or The BRAIN Initiative®. The Initiative had as its singular focus to develop and apply “innovative technologies to construct a dynamic picture of brain function that integrates neuronal and circuit activity over time and space.” Over the past decade, the BRAIN Initiative has exceeded all expectations by energizing and accelerating interdisciplinary research to map, monitor and modulate brain circuits. The BRAIN Initiative’s initial strategic plan, articulated in the BRAIN 2025 report, set bold scientific goals that would propel neuroscience far into the future. In November, across 21 articles, the BRAIN Initiative Cell Atlas Network (BICAN) published comprehensive cell atlases of adult and developing human and non-human primate brains. The groundwork is now set to create a comprehensive connectome of the human brain. Just last month, the BRAIN Initiative Cell Census Network (BICCN) unveiled a complete cell atlas of the mouse brain, a momentous step forward toward one of BRAIN’s goals to completely characterize a mammalian brain. The resulting data and resources are now available to the research community to empower functional studies about brain function and brain disorders in both human and animal models of neuro/mental/substance abuse disorders.
As highlighted in a recent message from NIH BRAIN Director Dr. John Ngai, the Initiative’s next transformative project, BRAIN Initiative Connectivity Across Scales (BRAIN CONNECTS) launched an initial set of awards to develop technologies to comprehensively map neural connections in both human and animal systems. Such “wiring diagrams” will be crucial for testing new hypotheses about how brain circuits function in health and disease. The BRAIN Initiative also continues to support innovative first-in-human trials using deep brain stimulation combined with artificial intelligence technologies that are showing great promise for the treatment of a variety of neurologic and neuropsychiatric disorders. In addition, the BRAIN Initiative is at the forefront of cross-cutting areas integral to advancing neuroscience research, including neuroinformatics, neuroethical considerations, and a commitment to enhance scientific excellence by increasing diversity and inclusivity in the research community.
Our commitment to basic science
NINDS support is unwavering for fundamental neuroscience (FN) research, which sets the foundation for discovery and progress against disorders of the nervous system. In 2023, the Fundamental Neuroscience Working Group (FNWG) of the National Advisory Neurological Disorders and Stroke (NANDS) Council, worked throughout much of the year to provide recommendations on how we can create opportunities to catalyze and accelerate discoveries in important fields of FN research. These efforts are essential as we seek new ways to stimulate innovation. As we now move to implement the WG’s recommendations, we do so in the context of the 2021-2026 NINDS Strategic Plan, which underscores the unique value of FN and our commitment to supporting investigator-initiated discovery research across the neuroscience spectrum.
2023 also saw the establishment of strategic priorities for amyotrophic lateral sclerosis (ALS) research(pdf, 1818 KB), through a NANDS Council Working Group comprised of scientists, clinicians, people living with ALS, people with genetic risk for ALS, and caregivers. We cannot underscore the urgency of this work enough, as ALS often occurs with no clear risk factors or family history, and the clock ticks quickly after symptom onset. As we address these strategic priorities, we also leverage important collaborations called for in the Accelerating Access to Critical Therapies, or ACT for ALS, including a Public-Private Partnership with the Food and Drug Administration (FDA) that includes multiple ALS non-profit organizations, the Critical Path for Rare Neurodegenerative Diseases (C-Path), the Foundation for NIH (fNIH), and multiple companies as partners in the effort to develop more effective treatments for persons with ALS. As part of this PPP, NINDS launched a national consortium of clinical research centers to carry out research within the PPP. I am especially grateful to those with lived experience of ALS that contributed to our strategic planning and the launch of the PPP, as their insights greatly enhanced and strengthened the Working Group’s recommendations.
The value of bolstered community engagement has informed other NINDS activities, including the culmination of a NANDS Council Working Group on Health Disparities and Inequities in Neurological Disorders. This past summer, the group published findings and recommendations from a two-year strategic planning effort for health disparities and equity research in a special issue of Neurology. The work reflects a wide range of perspectives on the elimination of disparities and inequities in neurological care, outcomes, treatment, access and provision of services, and research. I encourage you to learn more about our next steps, including our new Community-Engaged Health Equity Research in Neuroscience Initiative.
Leadership transitions
As seasons come and go, they also bring bittersweet changes. This year, we celebrated the career and retirement of Dr. Kurt Fischbeck, whose research has focused on the underlying mechanisms of hereditary neurological and neuromuscular diseases. His colleague, Dr. Michael O’Donovan, also entered retirement, after a long career in the field of spinal motor circuits. In addition, after 24 years, Dr. Bob Finkelstein has retired as Director of the NINDS Division of Extramural Activities, where he oversaw extramural research for the Institute. These colleagues made tremendous contributions to neuroscience, NINDS, and NIH, and I wish them well in their new life chapters.
Along with these transitions, we welcomed new faces and perspectives, including the appointment of Dr. Jeff Diamond as NINDS Scientific Director, who will provide overall executive direction and scientific leadership for the entire NINDS Intramural Research Program. He is joined by Dr. Faith Harrow Plante, who will direct the NINDS Office of Research, Training, and Career Development. Finally, I am excited for the leadership of new NIH Director Dr. Monica Bertagnolli, who transitions into the role after serving as Director of the National Cancer Institute. Moving into the new year, we look forward to a new NINDS Deputy Director – with endless thanks to Dr. Amy Bany Adams who has performed these duties in an acting capacity – and anticipate fresh vision and leadership to shepherd us into 2024.
As we await Fiscal Year (FY) 2024 budget information, NINDS is currently operating under a Continuing Resolution through February 2, 2024, at the FY 2023 enacted funding level. We anticipate implementing a conservative funding strategy during this time. While this news can be challenging, I have witnessed the resilience of this community time and time again, and feel certain that our tenacity will guide us through these more uncertain times. Together, we will continue to move forward in service of our mission: pursuing important work to gain a deeper understanding of the brain and nervous system to develop effective therapies for all people.









