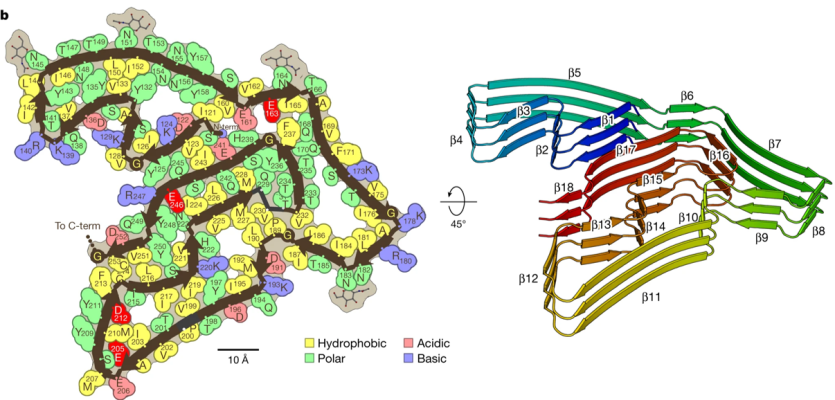
Amyloid fibrils in FTLD-TDP are composed of TMEM106B and not TDP-43
Researchers have known that amyloid fibrils composed of the protein TDP-43 are present in the brains of people with Alzheimer’s and Parkinson’s disease, and other neurodegenerative disorders. Now, researchers have discovered that in frontotemporal lobar degeneration (FTLD), instead of TDP-43, the aggregated fibrils are made up of the little-known protein TMEM106. In the study, they obtained postmortem samples from four people with FTLD and used cryogenic electron microscopy (or cryo-EM) to closely examine the protein structure. Whether TMEM106B contributes to FTLD is unknown but determining its structure will help inform the design of future therapeutics. This is one of several breakthroughs made this year in elucidating the structure of fibril proteins involved in Alzheimer’s disease and related dementias, including amyloid, alpha-synuclein, TDP-43, and TMEM (see below).
Image: The protein TMEM106B with its golf-course like fold (left). In FTLD, TMEM106B proteins are layered on top of one another to form amyloid fibrils (right).
Article: Jiang, Yi Xiao et al. Amyloid fibrils in FTLD-TDP are composed of TMEM106B and not TDP-43. March 28, 2022. Nature. https://pubmed.ncbi.nlm.nih.gov/35344984/
Additional articles: Schweighauser, Manuel et al. Age-dependent formation of TMEM106B amyloid filaments in human brains. March 28, 2022. Nature. https://pubmed.ncbi.nlm.nih.gov/35344985/
Yang, Yang et al. Structures of α-synuclein filaments from human brains with Lewy pathology. Sept 15, 2022. Nature. https://pubmed.ncbi.nlm.nih.gov/36108674/
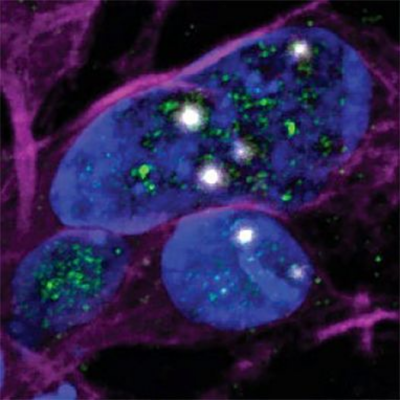
TDP-43 loss and ALS-risk SNPs drive mis-splicing and depletion of UNC13A
Scientists from two independent research teams discovered how the mislocalization of a protein, known as TDP-43, alters the genetic instructions for UNC13A and potentially other proteins, providing a possible therapeutic target that could also have implications in treating amyotrophic lateral sclerosis (ALS), frontotemporal dementia (FTD), and other forms of dementia. ALS and FTD are two neurodegenerative disorders in which many cases are linked by mislocalization of TDP-43, where instead of being primarily located in the nucleus of the cell where genes are activated, it forms aggregates outside the nucleus in multiple neurodegenerative diseases. Rare mutations in the TDP-43 gene are known to cause ALS, but almost all cases of ALS show mislocalization of TDP-43. Overall, these findings directly link a well-established risk factor for ALS and FTD with the loss of TDP-43. They also suggest that increasing the levels of UNC13A may be effective in preventing the death of neurons in these disorders.
Image: Cryptic exons (white) can be seen in cell nuclei (blue) in which there is little TDP-43 (green).
Articles:
Brown A-L et al. TDP-43 loss and ALS-risk SNPs drive mis-splicing and depletion of UNC13A. Nature. February 23, 2022. https://pubmed.ncbi.nlm.nih.gov/35197628/
Ma XR et al. TDP represses cryptic exon inclusion in the FTD-ALS gene UNC13A. Nature. February 23, 2022. https://pubmed.ncbi.nlm.nih.gov/35197626/
NIH Press Release: https://www.ninds.nih.gov/news-events/press-releases/scientists-discove…
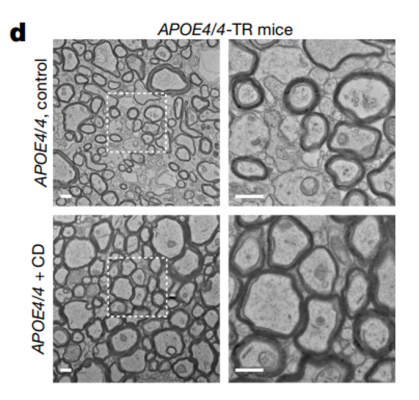
Cholesterol transport promotes new therapeutic opportunities for Alzheimer’s disease
Apolipoprotein E, specifically APOE4, is the strongest genetic risk of Alzheimer’s disease. The effects of APOE4 on the human brain are not fully understood, limiting the development of targeted therapeutics for Alzheimer’s disease. Researchers at the Massachusetts Institute of Technology found that APOE4 affected gene expression changes across all cell types in the human brain. The team also looked at genes related to cholesterol and found cholesterol-manufacturing genes were overly expressed, and that cholesterol-transporting genes dysregulated in brain cells called oligodendrocytes with the APOE4 gene. They also found that facilitating cholesterol transport, using cyclodextrins, increases myelination and improves cognitive function in APOE4 mice. These results suggest that cholesterol transport could help target impaired myelination and that the APOE genotype can inform new therapeutic and diagnostic opportunities for Alzheimer’s disease.
Image: Comparison of corpus callosum in APOE4 mice treated with saline (top) and cyclodextrin (bottom) indicating increased myelination in the cyclodextrin-treated group
Article: Blanchard, J. W. et al. APOE4 impairs myelination via cholesterol dysregulation in oligodendrocytes. November 16, 2022. Nature. https://pubmed.ncbi.nlm.nih.gov/36385529/
NIH Press Release: https://www.nih.gov/news-events/nih-research-matters/alzheimer-s-tied-c…
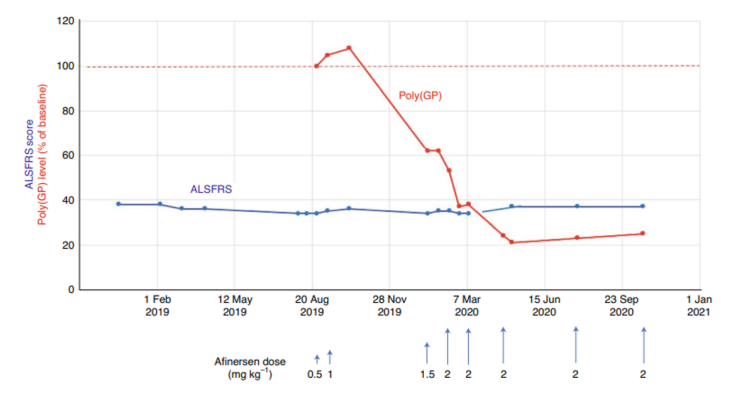
Feasibility of the clinical suppression of mutant C90RF72 expression
G4C2 repeat expansions in the C90RF72 gene are the most common genetic cause of two adult-onset neurodegenerative disorders, amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). Researchers at the University of Massachusetts Medical School used antisense oligonucleotides (ASOs) that decrease expression of G4C2 repeat-containing transcripts and effectively suppressed tissue levels of poly(GP) dipeptides. This research provides insight into the effect of nucleic acid chemistry on toxicity and, for the first time, demonstrates the feasibility of clinical suppression of the C90RF72 gene for potential new therapeutic strategies for ALS and FTD. Additional reading related to this study can be found below.
Image: Clinical summary of a patient’s Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS) score (blue line) and poly(GP) dipeptide levels (red line) during treatment with ASOs.
Article: Tran, H. et al. Suppression of mutant C9orf72 expression by a potent mixed backbone antisense oligonucleotide . December 23, 2021. Nature Medicine. https://pubmed.ncbi.nlm.nih.gov/34949835/
Additional article: Korobeynikov, V. A. et al. Antisense oligonucleotide silencing of FUS expression as a therapeutic approach in
amyotrophic lateral sclerosis. January 24, 2022. Nature Medicine. https://pubmed.ncbi.nlm.nih.gov/35075293/
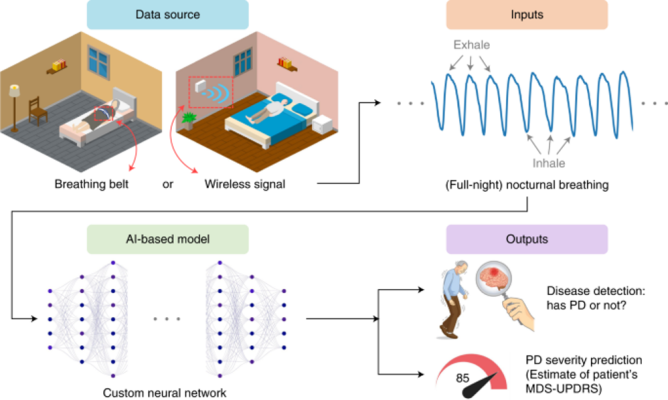
Artificial intelligence trained program to detect and track Parkinson’s disease by analyzing patients breathing patterns
NIH supported scientists have developed an Artificial Intelligence (AI) based system that can detect Parkinson’s disease (PD), assess its severity, and track its progression, while the patient is asleep. Traditionally, clinicians rely on a questionnaire called the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) to access PD progression, which is semi-subjective and lacks sensitivity to measure small changes in PD patients. By leveraging the relationship between PD and breathing, researchers trained an AI model to decipher breathing patterns of PD patients when they are sleeping at home. This study provides a promising AI application for biomarkers that can diagnose PD and track the severity of the disease, while also differentiating between PD and Alzheimer’s. In addition, the finding provides the potential of a biomarker that is noninvasive and can be assessed at home, potentially reducing the cost and duration of PD clinical trials.
Image: Overview of AI models to collect nocturnal breathing from PD patients for diagnosis and progression. Patient nocturnal breathing was assembled at home by wearable or wireless devices, then an AI trained system was used to analyze the breathing patterns that correlated with PD severity and progression.
Article: Yang, Yuzhe, et al., Artificial intelligence-enabled detection and assessment of Parkinson's disease using nocturnal breathing signal. October 2022. Nature Medicine. https://pubmed.ncbi.nlm.nih.gov/35995955/

NIH BRAIN Initiative aims to create the world's most comprehensive atlas of the human brain
The NIH Brain Research Through Advancing Innovative Neurotechnologies® (BRAIN) Initiative Cell Atlas Network (BICCN) received $500 million in funding over the next five years to help scientists understand how the human brain functions and how diseases affect it. One of BICAN's goals is to map and illustrate all neuronal and non-neuronal cells in the human brain, which comprise 200 billion cells. Another effort is to study epigenetic and genetic changes using a larger dataset from hundreds of human brains. Other related projects, including the BRAIN Armamentarium, will support the development of technologies, production efforts, and dissemination resources for a cell type-specific armamentarium to study brain function across species, as well as BRAIN CONNECTS, which will aim to enable mapping of mammalian brain connectivity across multiple species.
Image: Brain activity is orchestrated by propagating information between brain regions through fiber tracts, visualized via diffusion MRI tractography.
(Photo credit: Sahar Ahmad, Ye Wu, and Pew-Thian Yap, the University of North Carolina at Chapel Hill)
Link: Kaiser, J. NIH’s BRAIN Initiative puts $500 million into creating most detailed ever human brain atlas. September 22, 2022. Science. https://www.science.org/content/article/nihs-brain-initiative-puts-dollar500-million-creating-detailed-ever-human-brain-atlas#.Yy2kE0eLRuA.twitter
NIH Press Release: https://www.nimh.nih.gov/news/science-news/2022/nih-brain-initiative-la…
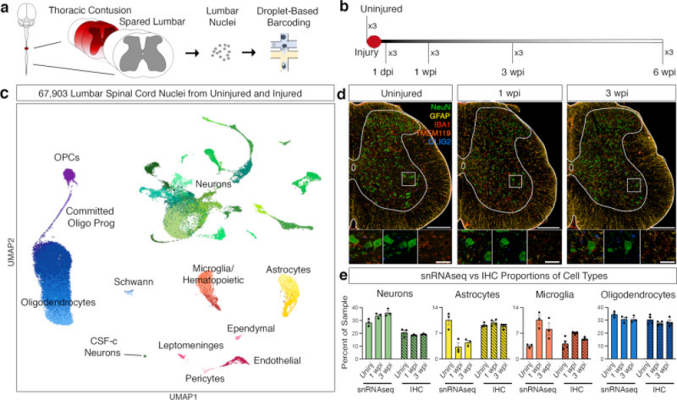
Scientists discover rare spinal neurons that express a signature of regeneration in response to spinal cord injury
Researchers have identified an endogenous cellular response by which the adult nervous system can recover from a severe spinal cord injury (SCI). Using single nucleus RNA Sequencing (snRNA-seq), researchers created a detailed map of lumbar cell types after injury and discovered a subset of neuronal populations that expressed regeneration-associated genes (RAGs) to promote recovery in SCI and observed the change of plasticity in spinocerebellar neurons after an injury. This work provides an essential resource for studying cellular responses to injury and uncovers the spontaneous plasticity of spinocerebellar neurons, uncovering a potential candidate for targeted therapy.
Image: Single nucleus RNA sequencing of the lumbar spinal cord after thoracic contusion. a Schematics depicting the experimental design for snRNA-seq, showing the injured thoracic cord and lumbar cord (with dark red representing the lesion) as well as nuclei isolation from the intact lumbar cord followed by droplet-based barcoding for single nucleus RNA sequencing. b Top, an overview of experimental design for injury and tissue collection. The lumbar spinal cord of three animals from each time point: uninjured, 1 dpi (day post injury), 1 wpi (week post injury), 3 wpi, and 6 wpi. c Uniform manifold approximation and projection (UMAP) visualization of 67,903 nuclei from uninjured and injured lumbar spinal cords, revealing 9 classes and 39 subtypes. Colored by green (neurons), yellow, astrocytes, orange-red (microglia), purple (OPCs), blue (oligodendrocytes), light blue (Schwann), light pink (pericytes), pink (ependymal), magenta (leptomeninges), brick-red (endothelial). d Multiplex immunohistochemistry (IHC) of the lumbar spinal cord from uninjured, 1 wpi and 3 wpi. Tissue was stained for NeuN (green), GFAP (yellow), IBA1 (red), TMEM119 (dark orange), and OLIG2 (blue). Scale bars are 200 µm (main) and 50 µm (inset), respectively. e Quantification of the proportion of cell types from the snRNA-seq data and immunohistochemistry in tissue. Mean ± SEM; snRNAseq, N = 3; immunohistochemistry, N = 4.
Article: Matson, K.J.E., et al. Single cell atlas of spinal cord injury in mice reveals a pro-regenerative signature in spinocerebellar neurons. September 26, 2022. Nature Communications. https://pubmed.ncbi.nlm.nih.gov/36163250/
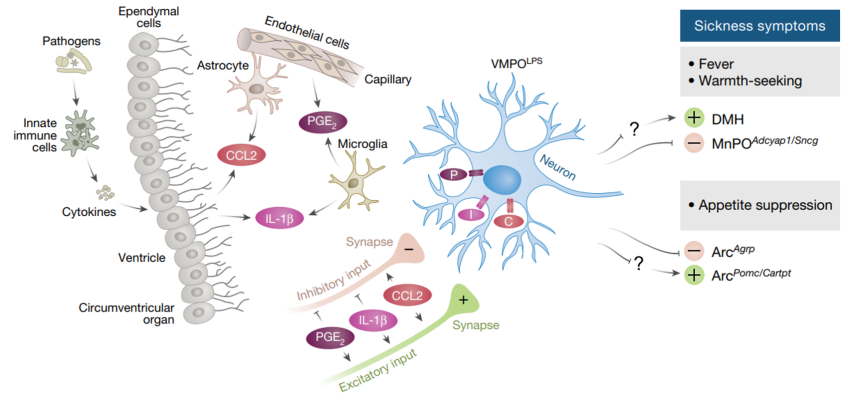
A preoptic neuronal population controls fever and appetite during sickness
Fatigue, loss of appetite, and changes in temperature occur when the immune system responds to infections, autoimmune disorders and a host of other conditions. Researchers led by Dr. Catherine Dulac at Harvard University identified a population of neurons in mice brains that are involved in responding to inflammation. To induce sickness, the researchers injected mice with an agent that triggers an immune, which activated cells in a part of the hypothalamus called the ventral medial preoptic area (VMPO). Using chemogenetic and optogenetic tools, the team was able to determine that this population of neurons is important for regulating body temperature and appetite. Additionally, their findings indicate that this population of neurons sends signals to brain areas associated behavioral and homeostatic functions.
Image: Proposed model of VMPOLPS neuron control of body temperature and appetite. Activation of the immune system in the periphery leads to circulating immune signals. These signals activate cells lining the blood–brain barrier, which react by secreting additional signals, which are further amplified by local glial cells. VMPOLPS neurons are activated by these signals and induce sickness symptoms such as fever, warmth seeking, and appetite suppression.
Article: Osterhout, J.A. et al. A preoptic neuronal population controls fever and appetite during sickness, June 8, 2022, Nature.
https://pubmed.ncbi.nlm.nih.gov/35676482/
NIH Research Matters Article: https://www.nih.gov/news-events/nih-research-matters/brain-cells-contro…
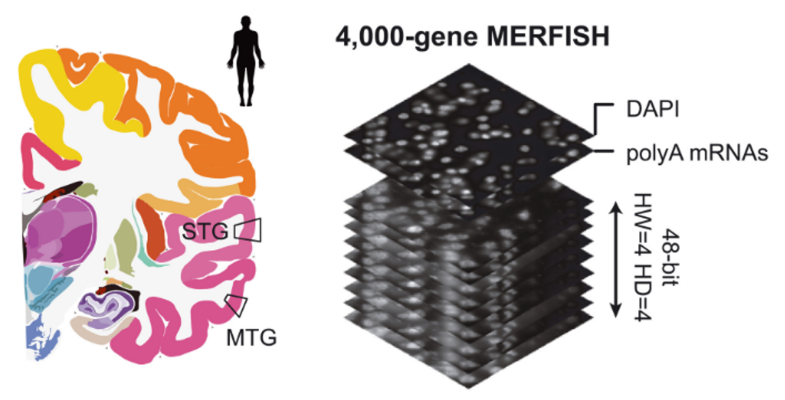
Conversation and divergence of cortical cell organization in human and mouse revealed by MERFISH
Researchers led by Dr. Xiaowei Zhuang at Harvard University conducted a study using MERFISH, an imaging technique, to analyze the distribution of cell types within the human and mouse cortexes. Using 4,000 gene transcripts in their analysis, they identified patterns of cell-type-specific associations that differed between the two species. Their analysis revealed that mouse and human contain similar types of neurons but the connections between them are more numerous and complex in the human. The human brain has many more specific neurons that are closely located to and interacting with other similar neuronal cell types. The researchers also investigated receptor-ligand pairs and discovered potential connections between neurons and microglia that may help explain the link between certain genes and neurodegenerative diseases.
Image: A schematic representation of 4000-gene MERFISH measurements of the human middle temporal gyrus and superior temporal gyrus using a 48-bit error-correcting code.
Article: Fang R and Xia C et al. Conservation and divergence of cortical cell organization in human and mouse revealed by MERFISH, June 30, 2022, Science.
https://pubmed.ncbi.nlm.nih.gov/35771910/
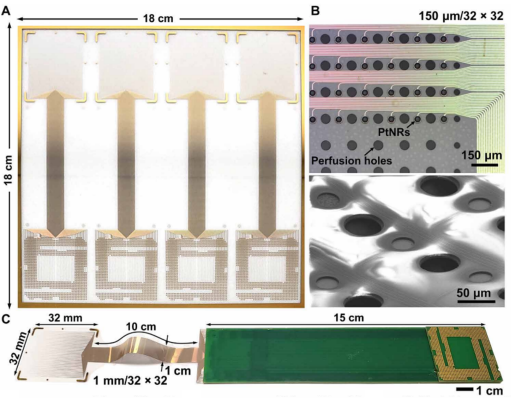
Human brain mapping with multithousand-channel PtNRGrids resolves spatiotemporal dynamics
Researchers led by Shadi Dayeh at the University of California San Diego created a scalable manufacturing process for constructing reconfigurable thin-film neurophysiological recording grids with a dense electrical connection scheme using platinum nanorods (PtNRGrids). PtNRGrids allowed for a multi-thousand-channel array of small contacts that enabled high spatial and temporal resolution over a large area of the surface of the brain. When applied to rodents, PtNRGrids were able to resolve submillimeter functional organization of the barrel cortex. In the clinic, PtNRGrids were able to resolve complex temporal dynamics of awake human patients, as well capture spatial dynamics of epileptic discharges during surgery.
Image: Multithousand-channel platinum nanorod grid electrocorticogrpahy arrays.
Article: Tchoe Y, Bourhis AM, and Cleary DR et al. Human brain mapping with multithousand-channel PtNRGrids resolves spatiotemporal dynamics. Jan 19, 2022, Sci Transl Med.
https://pubmed.ncbi.nlm.nih.gov/35044788/
Each new year offers a clean slate, with an opportunity to reflect on lessons learned as we set our eyes on new goals and possibilities. In 2022, we experienced a third year of the COVID-19 pandemic and also saw society slowly returning back to “normal” as most restrictions lifted in the United States and across the world. For many who suffer with neurological disorders, challenges associated with the pandemic persist. Yet, I have seen our research community demonstrate resilience each and every day to pursue scientific questions that offer hope to those who lack effective treatments. I see this resilience at home, where our intramural community has been diligently working with buildings and facilities staff to recover and resume research after a building flood, a result of the December Arctic blast. This effort continues, and as we witness their compassion, patience, and resolve, we cannot help but feel optimism and hope for 2023.
We continue to learn more about the acute and long-term effects of a COVID-19 infection, known as the post-acute sequelae of SARS-CoV-2 infection (PASC), including Long COVID. NINDS investigators reviewed the neurological symptoms associated with acute COVID-19, and studied the impact of a COVID-19-triggered immune response on the brain. In addition, for the past two years, NIH’s National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Allergy and Infectious Diseases (NIAID), and NINDS, along with several other NIH institutes and the office of the NIH Director, have been leading NIH’s Researching COVID to Enhance Recovery (RECOVER ) initiative, a national research program to understand PASC. I wrote about this effort on the NIH Director’s Blog, noting that these efforts will help us recognize the full range of outcomes and needs resulting from PASC and, most importantly, understand how to enable people to make a full recovery from COVID-19.
This past year, NINDS continued to support investigators who – even as we continued to endure a global pandemic – persevered and achieved innovative advances in basic and clinical neuroscience. While I cannot include all of them here, I have selected a few highlights that you can view above. The past year also brought us opportunities to explore and build new research programs, collaborations, and partnerships, especially through our trans-NIH Initiatives. In recognition of the urgent need to improve the management of pain, NINDS has been a leader in trans-NIH pain research efforts and opportunities including the Helping to End Addiction Long-term® Initiative, or NIH HEAL Initiative®, and this year’s annual symposium of the NIH Pain Consortium, which examined pain management through the lens of whole person health. NINDS also continues to play a critical role in the NIH BRAIN Initiative, which announced two transformative projects to improve our understanding of brain cell types and develop precise tools needed to access them.
In 2022, we marked the 10th anniversary of the National Action Plan for Alzheimer’s Disease, which along with increased funding from Congress, has enabled ambitious new research on Alzheimer's Disease and Alzheimer's Disease Related Dementias (AD/ADRD). NINDS also held the fourth triennial ADRD Summit to set updated research priorities to build on this momentum. FDA’s recent accelerated approval of an anti-amyloid therapy brings a disease-modifying therapy for people with early signs of Alzheimer’s Disease. The new anti-amyloid antibody treatment, however, raises a new challenge for our basic and clinical scientists: to understand how to prevent the treatment’s propensity to injure the blood-brain barrier.
Most NINDS meetings continued to be held virtually this past year. While these virtual venues are no replacement for engaging in-person interactions, they do enable greater reach and attendance at critical meetings and events, including this year’s NINDS Nonprofit Forum and 8th annual BRAIN Initiative Meeting. We also leveraged virtual gatherings to solicit valuable community input and collect feedback from experts in the field, such as through the Alzheimer's Disease-Related Dementias Virtual Summit, a symposium of the Brain Attack Coalition about expanding access to life-saving acute stroke therapy and our Amytrophic Lateral Sclerosis (ALS) Strategic Planning Workshop, the results of which will be presented to our Advisory Council on February 1. Still, I have missed in-person meetings, and am optimistic that their return will foster invaluable opportunities for connecting and collaborating across the neuroscience and neurological disorders community. Indeed, I was thrilled to meet patients and their families at the Global Foundation for Peroxisomal Disorders’ Family and Scientific Conference, as well as many scientists at the first in-person annual meeting of the Society for Neuroscience (SfN) since 2019, which convened thousands of neuroscientists in San Diego, California for fist bumps, hallway chats, and in-person conversations. NINDS continues to closely monitor COVID-19 risk levels as it is now slowly and safely transitioning back to in-person meetings and gatherings, while also enabling virtual capabilities based on our lessons learned over the last three years.
Ensuring a vibrant, talented, and diverse neuroscience workforce is just one of the priorities outlined in our 2021-2026 NINDS Strategic Plan, and I am proud of NINDS’ longstanding contributions to effective and creative programs that support workforce diversity. In 2022, we continued efforts catalyzed by the events of 2020 toward improved workforce diversity. We developed a Racial and Ethnic Equity Plan (REEP) for NINDS, part of a broader NIH initiative to enhance the diversity of our workforce and to identify and dismantle racial and ethnic disparities. In addition, our CORE IDEALS – a Culture of Respect and Engagement through Inclusion, Diversity, Equity, Accessibility, and Leadership Support – are helping us to identify and implement strategies to create a workplace that is diverse and inclusive and free of harassment and discrimination. This year, we also developed a Diversity R01 for New and "At-Risk" Investigators from diverse backgrounds, including those groups underrepresented in the biomedical sciences in order to enhance the diversity of R01-funded investigators in the extramural workforce.
As I have written previously, NINDS is committed to promoting broad and appropriate sharing of scientific data to accelerate progress and the application of research findings. Prospectively planning for how scientific data will be managed and shared is a crucial step in optimizing the impact of data generated from NIH-funded research. Over the past year, NIH has been preparing to implement its Data Management and Sharing Policy (DMSP), which goes into effect January 25, 2023. I am grateful to NINDS staff who have worked to create a new website landing page with comprehensive information and frequently asked questions (FAQs) on how the DMSP will impact NINDS applicants. I encourage you to read through the resources that they have developed. Our challenge for the future is to devise means to make these data findable, accessible, interoperable and reusable (i.e., FAIR principles), so that they are not just posted, but contribute to advancing science.
Every year also brings transitions. After serving as NINDS Deputy Director and Acting NINDS Scientific Director, Dr. Nina Schor was selected as the next NIH Deputy Director for Intramural Research, taking on the challenge of leading the NIH intramural program. We miss her at NINDS but take comfort in knowing that she will drive excellence at a broader NIH level. To continue the important work of the NINDS Intramural Program, I appointed Dr. Jeffrey Diamond as Acting Scientific Director, and I am grateful for his leadership as we search for a permanent Scientific Director. I also appointed Dr. Amy Adams to Acting Deputy Director for NINDS, a leadership role to which she brings her broad NIH experience and thoughtful input. Finally, I am immensely thankful for the leadership, resilience, and steadfastness of Dr. Anthony Fauci, a fellow Brooklynite, who retired from his role as Director of the National Institute of Allergy and Infectious Diseases (NIAID) Director after 54 years of public service.
In reflecting on the struggles and progress that we have experienced over the last three years, it is clear that our patience and strength have been tested in many ways. Yet we, as a community of persons with neurological disorders and their caregivers, scientists and science administrators, have learned, endured, and advanced basic and clinical neuroscience, and this gives me optimism and hope for all that 2023 will bring. To that end, we begin the year with a 7.76% increase in the NINDS appropriated budget over the Fiscal Year 2022 enacted level, which includes $225 million from the NIH Innovation account for the NIH BRAIN Initiative. Among other items, the budget will also include $75 million for the Accelerating Access to Critical Therapies for ALS Act, $18 million to fund the Undiagnosed Diseases Network, and an increase of $226 million for AD/ADRD research (with an increase of $75 million for NINDS).
Through it all, we will continue our important work to gain a deeper understanding of the brain and nervous system to develop effective therapies for all people.










