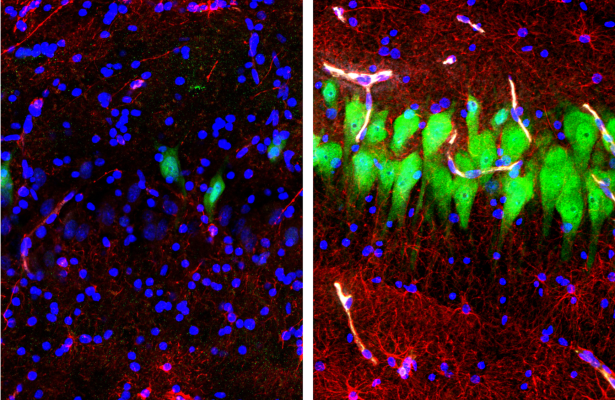
Researchers have developed a high-tech support system that can keep a large mammalian brain from rapidly decomposing in the hours after death, enabling study of certain molecular and cellular functions. The NIH BRAIN Initiative-funded researchers developed a way to deliver an artificial blood supply to the isolated postmortem brain of a pig, preventing the degradation that would otherwise destroy many cellular and molecular functions and render it unsuitable for study. Importantly, although the researchers saw some preservation of flow through blood vessels and energy use, there was no higher-level functional activity in the brain circuits. The new technology opens up opportunities to examine complex cell and circuit connections and functions that are lost when specimens are preserved in other ways.
Image: Immunofluorescent stains for neurons (NeuN; green), astrocytes (GFAP; red), and cell nuclei (DAPI, blue) in the hippocampal CA3 region of brains either unperfused for 10 hours after death (left) or subjected to perfusion with the BrainEx technology (right). After 10 hours postmortem, neurons and astrocytes normally undergo cellular disintegration unless salvaged by the BrainEx system.
NINDS/NIMH Press Release (April 17, 2019):
https://www.ninds.nih.gov/News-Events/News-and-Press-Releases/Press-Releases/NIH-BRAIN-Initiative-tool
Article: Vrselja Z et al., Restoration of brain circulation and cellular functions hours postmortem. Nature. April 18, 2019.
https://pubmed.ncbi.nlm.nih.gov/30996318-restoration-of-brain-circulation-and-cellular-functions-hours-post-mortem/
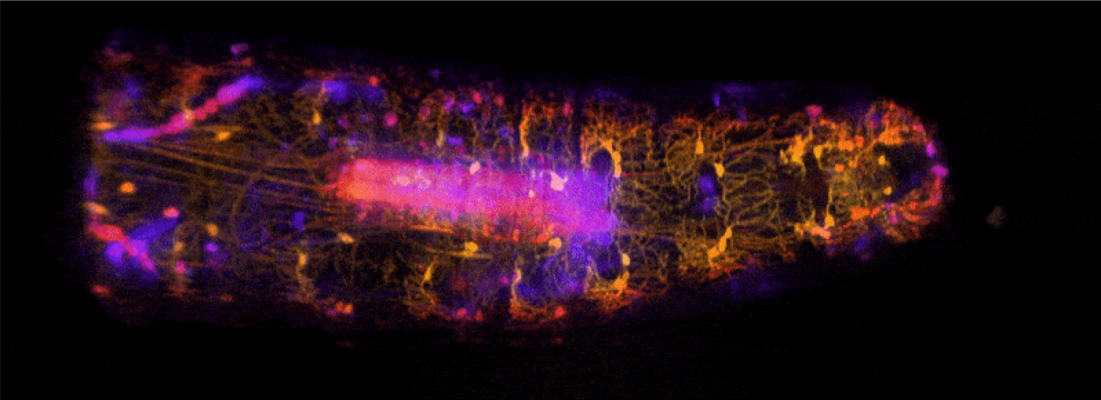
Dr. Elizabeth Hillman and colleagues at Columbia University utilized their previously-developed multispectral, high-speed, volumetric swept confocally aligned planar excitation (SCAPE) microscope to characterize proprioceptor system dynamics in live, freely moving Drosophila larvae. This approach allowed the group to examine real-time spatiotemporal and functional dynamics in Drosophila proprioceptors as the larvae contracted and extended segments during crawling, as well as during exploratory head movements. By investigating how a range of proprioceptive neurons can encode forward locomotion and exploration behavior during naturalistic movement, this finding holds important implications for models of sensory feedback, including understanding the neural mechanisms underlying movement in freely behaving animals.
Image: High-speed, volumetric SCAPE microscopy was used to capture the activity of proprioceptive neurons inside freely moving Drosophila larvae. The high-resolution imaging approach allowed researchers to view a continuum of sensory feedback from proprioceptive neurons during forward crawling and exploratory head movements. Central structure (in pink) is the larva’s ventral nerve cord.
No NINDS Press Release, but BRAIN Publication RoundUp:
https://brainupdate.nih.gov/2019/03/28/brain-publication-roundup-march-2019/
Article: Vaadia RD et al., Characterization of Proprioceptive System Dynamics in Behaving Drosophila Larvae Using High-Speed Volumetric Microscopy. Current Biology. March 18, 2019.
https://pubmed.ncbi.nlm.nih.gov/30853438-characterization-of-proprioceptive-system-dynamics-in-behaving-drosophila-larvae-using-high-speed-volumetric-microscopy/
Scientists develop resource for interrogating molecular and cellular basis of Alzheimer’s disease
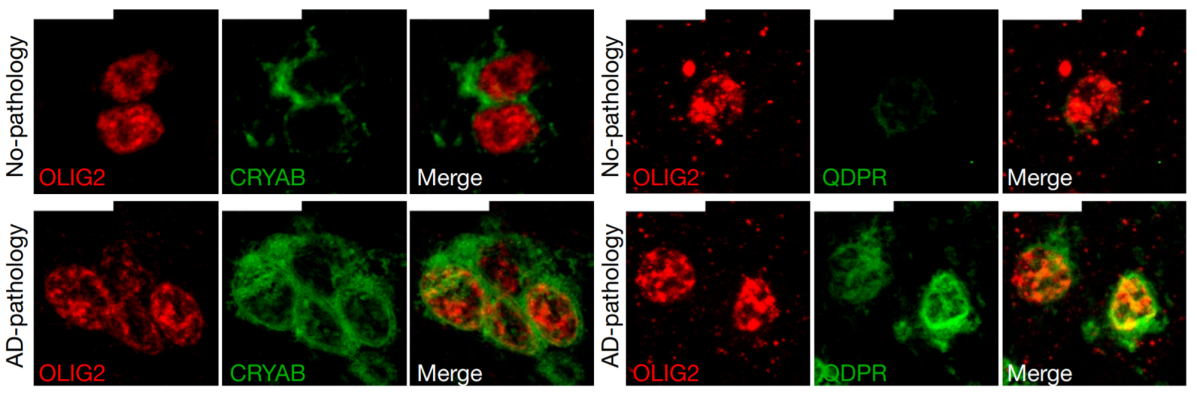
NINDS and other NIH Institutes including NIA, NIMH, and NHGRI, are invested in tackling Alzheimer's Disease (AD) and AD-related dementias. Recently, researchers interrogated the molecular and cellular basis of Alzheimer's disease using single-cell transcriptomic analysis. They analyzed over 80,000 single-nucleus transcriptomes from the prefrontal cortex of 48 individuals with varying degrees of AD pathology. Disease-related changes appeared early in pathological progression and were often cell-type specific, whereas late stage changes occurred across all cell types. Myelination-related processes were consistently altered across cell types, suggesting a key role for myelination in AD pathophysiology.
Image: Immunohistochemistry on oligodendrocytes in the white matter of Brodmann area 10 for individuals with no AD pathology (top row) or AD pathology (bottom row). These cell subtypes expressed high levels of QDPR and CRYAB. The latter is an anti-apoptotic and neuroprotective chaperone, the dysfunction of which could exacerbate inflammation and demyelination.
No NINDS Press Release
Article: Mathys H et al., Single-cell transcriptomic analysis of Alzheimer's disease. Nature. June 2019. https://pubmed.ncbi.nlm.nih.gov/31042697-single-cell-transcriptomic-analysis-of-alzheimers-disease/
Single-nucleus RNA-sequencing analysis reveals conserved cell types with divergent features in human versus mouse cortex
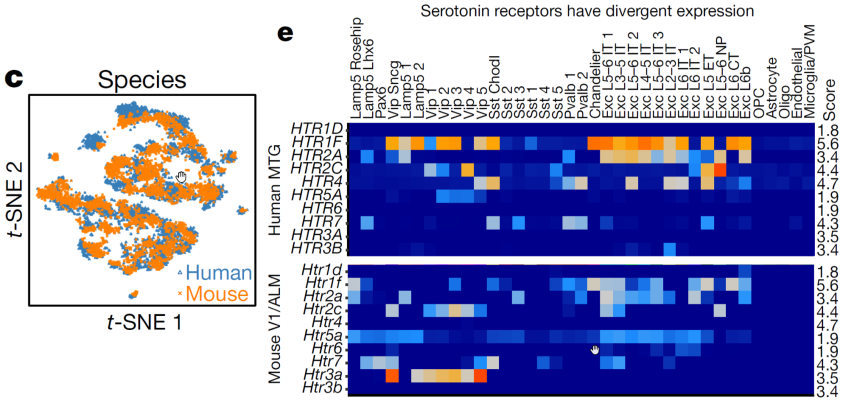
Researchers at the Allen Institute for Brain Science used single-nucleus RNA-sequencing analysis to perform a comprehensive study of cell types in middle temporal gyrus of human cortex. Comparison of this human cortex to similar mouse cortex datasets revealed well-conserved cellular architecture that enabled matching of homologous types and predictions of properties of human cell types. However, there were also extensive differences between homologous human and mouse cell types, including marked alterations in proportions, laminar distributions, gene expression and morphology. Despite cellular properties that were conserved across species, species-specific features emphasize the importance of directly studying human brain.
Image: (left panel) A visualization of human (blue) and mouse (orange) inhibitory neuron clusters, demonstrating a surprising degree of conservation across species. (right panel) Divergent cell-type expression is shown through expression of serotonin receptors across human (top) and mouse (bottom).
No NINDS Press Release
Article: Hodge RD et al., Conserved cell types with divergent features in human versus mouse cortex. Nature. September 5, 2019. https://pubmed.ncbi.nlm.nih.gov/31435019-conserved-cell-types-with-divergent-features-in-human-versus-mouse-cortex/
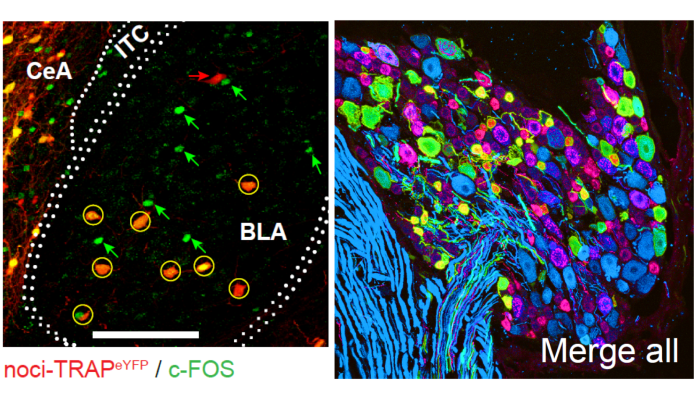
Pain is unpleasant. But how the brain’s emotional and neural circuits link the negative feeling of pain to nociceptive information is unclear. In this study, NINDS-funded researchers used in vivo calcium imaging via a miniaturized microscope and neural activity manipulation in mice to identify a group of neurons in the basolateral amygdala that are linked to these negative emotions. Importantly, silencing these neurons during painful stimuli (e.g., very hot or cold water) reduced emotional reactions to pain without affecting reflexes, anxiety, or reward-related behaviors. Dr. GrégoryScherrer and his team also found that these neurons were needed for experiencing the unpleasantness of neuropathic pain - a chronic pain condition. In the future, as an alternative to opioid treatment, scientists could target these neurons to treat both acute and chronic pain without the risk of addiction.
Image: Immunofluorescent image of nociceptive basolateral amygdala (BLA) neurons (red) that were re-activated (c-Fos; green) following a second pin prick to the paw (left). Once identified, these neurons were silenced using chemogenetics. Neurons expressing ChR2-eYFP (green) in peripheral nociceptors (red) of dorsal root ganglia of the spinal cord (right).
Article: Corder G et al., An amygdalar neural ensemble that encodes the unpleasantness of pain. Science. January 18, 2019.
https://www.ncbi.nlm.nih.gov/pubmed/30655440
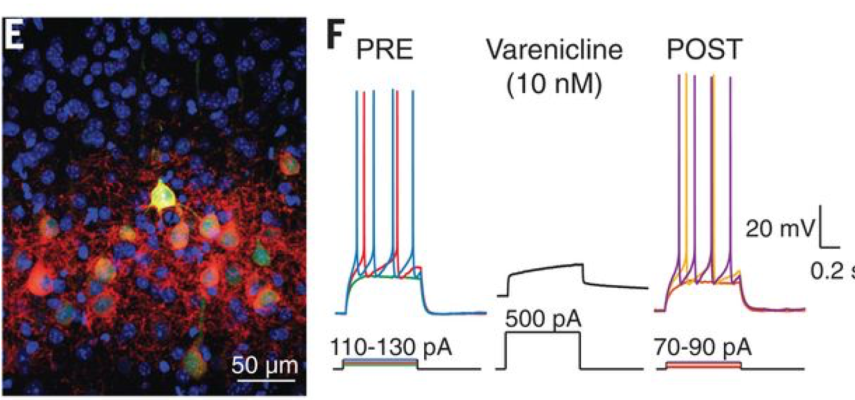
Chemogenetics is a popular method used to control the cellular activity of defined cell populations with a receptor engineered to respond selectively to a small molecule agonist or drug, thereby activating or inhibiting neurons. Chemogenetic tools have been widely used in animal models, and scientists are interested in developing this method for human therapeutics. Dr. Scott Sternson and his colleagues engineered ion channels that can be activated by low doses of the FDA-approved anti-smoking drug varenicline (i.e. Chantix(R)). After improving the drug’s potency and selectivity, researchers successfully used it to silence neurons in mice and non-human primates, and induced behavioral changes. This work is a proof-of-concept finding that this receptor-agonist combination is effective across species and importantly, brings chemogenetics one step closer to human therapeutic applications.
Image: Fluorescent image of mouse cortical neurons expressing the varenicline receptor PSAM4-GlyR (EGFP; green) with intact cell surface (red) (left). Varenicline (10nM) suppressed the firing of these neurons in vitro (right).
Article: Magnus CJ et al., Ultrapotent chemogenetics for research and potential clinical applications. Science. April 19, 2019.
https://www.ncbi.nlm.nih.gov/pubmed/30872534
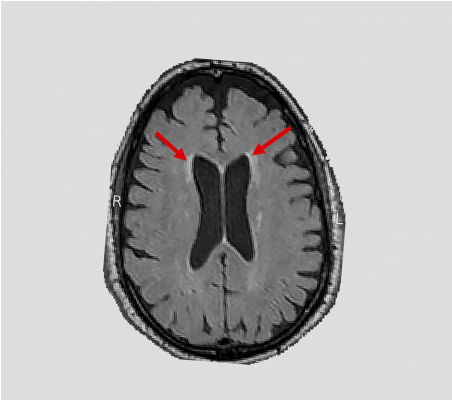
Hypertension (i.e., high blood pressure) is a primary risk factor for the formation of white matter lesions. Studies suggest that these lesions are associated with cognitive decline as well as Alzheimer’s and related dementias. Despite its proven cardiovascular benefits, how intensively controlling blood pressure affects brain health is unknown. In a nationwide study, NIH-funded researchers (supported by NHLBI, NIDDK, NIA, and NINDS) used magnetic resonance imaging (MRI) to scan the brains of hundreds of hypertensive participants in the NIH Systolic Blood Pressure Intervention Trial (SPRINT). They found that compared to standard blood pressure treatment, intensive blood pressure treatment reduced the accumulation of white matter lesions. These results complement a prior study which showed that this aggressive treatment lowered the risk of mild cognitive impairment. Taken together, these studies highlight the benefits that intensively controlling blood pressure may have on the brain and shine light on our understanding of aging brain disorders.
Image: An NIH-funded clinical trial - Systolic Blood Pressure Intervention Trial (SPRINT) - found a link between blood pressure and white matter lesions. Arrows highlight examples of lesions seen on magnetic resonance imaging brain scans.
NIH News Release (August 13, 2019):
https://www.nih.gov/news-events/news-releases/intensive-blood-pressure-control-may-slow-age-related-brain-damage
Article: Nasrallah, IM et al., Association of intensive vs standard blood pressure control with cerebral white matter lesions. Jama. August 13, 2019.
https://www.ncbi.nlm.nih.gov/pubmed/31408137
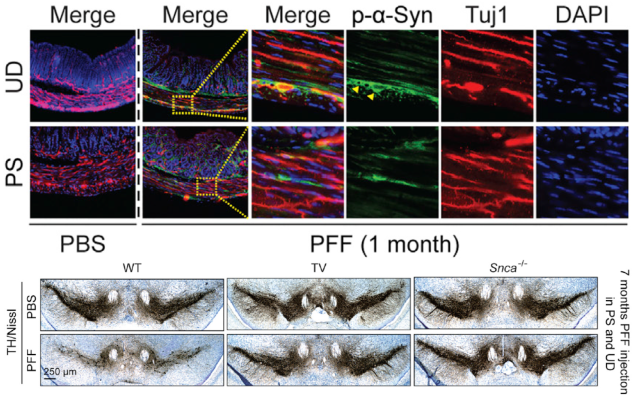
Neurodegenerative diseases - including Parkinson’s disease (PD) - are characterized by a buildup of a misfolded protein called alpha-synuclein (alpha-syn) in the brain. Evidence suggests that alpha-syn spreads from the gut to the brain via the vagus nerve, but this has yet to be proven. To test this theory, NINDS-funded scientists injected synthetic alpha-syn into the guts of healthy mice. Over the course of 10 months, they found that the alpha-syn spread from one brain region to the next in a predictable pattern that mimicked neurodegeneration seen in human PD patients. Dr. Han Seok Ko and his colleagues also showed that they could prevent this gut-to-brain spread and associated behavioral dysfunction by severing the vagus nerve. These results may offer a new, more precise method to test treatments that could prevent or halt PD in humans.
Image: Immunofluorescent image of gut tissue stained for pSer129-a-syn (green) and Tuj-1 (red) – indicators of alpha-syn presence – in the upper duodenum (UD) and pyloric stomach (PS) one-month after alpha-syn injection (top). Photomicrographs from mesencephalon sections containing dopamine (TH-positive) neurons in the substansia nigra pars compacta region seven months after alpha-syn injection into the guts of a healthy (left), vagotomized (middle), and alpha-syn knockout mouse (right) (bottom).
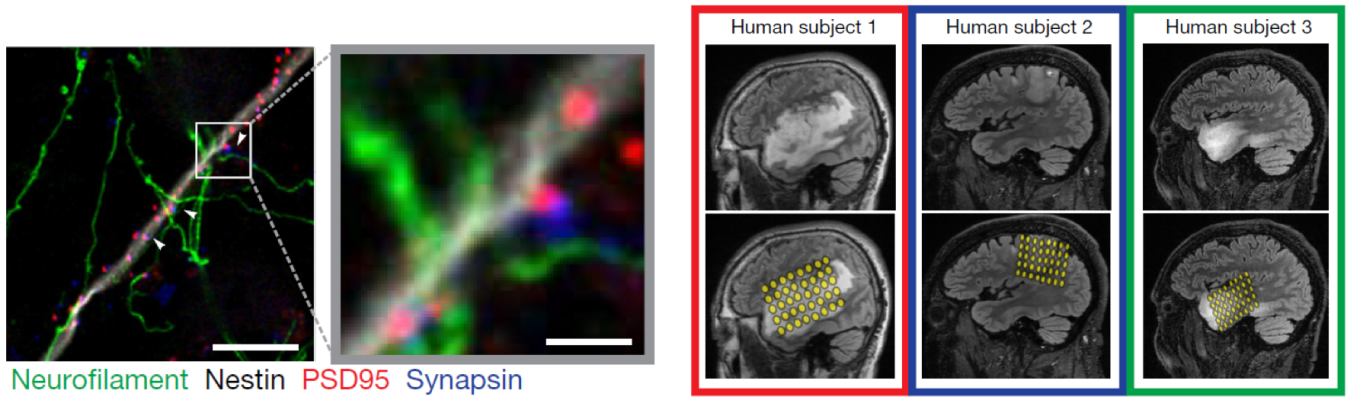
High-grade gliomas are lethal brain cancers whose progression is driven by neural activity, but precisely how neighboring neurons in the microenvironment promote glial growth is unknown. By imaging mouse brain tissue with cells from human gliomas via electron microscopy, Dr. Michelle Monje and her team identified synaptic connections between neurons and gliomas. Researchers also showed that these connections were electrochemical and that interrupting electrical signals between high-grade gliomas and healthy neurons with anti-epilepsy drugs greatly reduced tumor growth in mice. Further, they demonstrated that neurons near gliomas were hyperexcitable in humans. This NINDS-funded study may pave the way for novel tumor treatment approaches for patients with gliomas.
Image: Fluorescent image of synaptic connections between gliomas and neurons in a mouse brain. White box and arrows highlight a region with dense synaptic connections. Green denotes neurofilament (axon); white denotes glioma cell processes; blue denotes synapsin (presynapse); red denotes glioma postsynaptic puncta.
Article: Venkatesh, HS et al., Electrical and synaptic integration of glioma into neural circuits. Nature. September 26, 2019.
https://www.ncbi.nlm.nih.gov/pubmed/31534222

Vascular risk factors, including consuming high levels of dietary salt, are associated with cerebrovascular disease and cognitive impairment. In a recent study, Dr. Contantino Iadecola and his team showed that mice eating a diet high in sodium began to show symptoms of dementia due to changes in the gut. Mice also developed reduced cerebral blood flow, but researchers suspected that this hypoperfusion was not linked to cognitive dysfunction. Instead, they showed that dietary salt induces tau phosphorylation and cognitive decline in middle aged mice, and that these effects are prevented by restoring normal gut function. Moreover, tau knockout mice on a high salt diet showed reduced blood flow, but no tau aggregates nor cognitive impairments. This NINDS-funded study highlights the importance of considering nuanced factors, such as diet and gut health, in preventing the neurodegeneration that underlies dementia.
Image: Images showing increased neuronal AT8 immunoreactivity – a marker of increased tau phosphorylation – in the piriform cortex of mice on a normal (ND) and high-salt (HSD) diet. Right images are magnified views of the white boxes on the left.
NIH News Release (October 23, 2019):
https://www.nih.gov/news-events/news-releases/pathogenic-tau-cognitive-impairment-are-precipitated-high-salt-diet
Article: Faraco, G et al., Dietary salt promotes cognitive impairment through tau phosphorylation. Nature.
https://www.ncbi.nlm.nih.gov/pubmed/31645758
The end of a decade – and the start of a new one – brings a certain state of reflection that can differ from the usual year-to-year perspective. As I look back on the last decade, and particularly in the last year, I am heartened and encouraged by the significant strides that NINDS has made and continues to make through funding awards, facilitating collaborations, launching new initiatives, and transitioning leadership roles. With gratitude, we thank our investigators, research subjects, and our partners representing those suffering from neurological disorders and stroke.
In 2019, NINDS launched its next strategic planning effort, led by NINDS Deputy Director Dr. Nina Schor, which will help to set overarching goals for the institute to achieve in the next 5-10 years. For this effort, we aim to tune our practices and policies to our vision and mission, in order to better serve and anticipate the needs of the research and patient communities and the public. We want to dream big! As a first step for this process, we conducted an initial request for information (RFI) to inform the roadmap by which internal NINDS taskforces will collaborate with both internal and external stakeholders. While the RFI submission deadline has passed, we continue to monitor submissions and want to hear from you! Please also continue to follow along as we post new opportunities for engagement during the strategic planning process.
One of the biggest 2019 accomplishments was the announcement of the awards for the Helping to End Addiction Long-term Initiative, or NIH HEAL Initiative, investing $945M in research across approximately 375 awards in 41 states. The HEAL Initiative presents an unprecedented opportunity to advance the field of pain research and reduce reliance on opioid medications. As the lead institute for pain research at NIH, NINDS helped to develop the pain management pillars of the Initiative and will serve as the hub for several programs to accelerate the development of new therapies. One notable program is the Early Phase Pain Investigation Clinical Network (EPPIC-Net) which provides the infrastructure and clinical population for early stage clinical trials to test non-addictive pain therapies. Applications for trials to be conducted in EPPIC-Net are open and evaluated on a rolling basis. As NIH staff have worked tirelessly to ensure the quick launch of these awards, I thank them and am eager to follow the progress of these programs to improve pain management and outcomes through research.
At its halfway point, the BRAIN Initiative is revolutionizing neuroscience research. Since 2014, NIH has invested over $1.3 billion in the BRAIN Initiative, supporting over 700 awards to hundreds of investigators. These awards leverage broad scientific disciplines and both investigator- and team-based science towards addressing fundamental questions in neural circuit function, and to date, hundreds of publications have generated new tools and neurotechnologies that better help us see the brain in action. Numerous events, including most recently at the 2019 Annual Meeting of the Society of Neuroscience, highlighted the game-changing nature of these tools and resources, as they begin to be distributed to the wider basic neuroscience community and impact clinical research.
As the NIH BRAIN Initiative moves toward its second half, I am thrilled it is doing so under the leadership of Dr. John Ngai, recently appointed as NIH BRAIN Initiative Director, who will join the NIH team in March. Dr. Ngai is currently the Coates Family Professor of Neuroscience at the University of California, Berkeley, and has built a long-standing research program on how the nervous system detects odors and turns them into neural signals sent to the brain. He will oversee both day-to-day operations and BRAIN’s long-term strategy, which is informed by two recently accepted reports on the progress and emerging opportunities in neuroscience, led by Drs. Catherine Dulac and John Maunsell, and neuroethics, led by Drs. Jim Eberwine and Jeff Kahn, for the Initiative. I thank both working groups for their diligent efforts in this strategic planning process over the last year and a half, and with their discussions and under the helm of Dr. Ngai, I am excited for what scientific advances from BRAIN are just around the corner.
Another notable 2019 accomplishment is the launch of the Accelerating Medicines Partnership (AMP) Parkinson’s Disease (PD) Knowledge Portal. This platform will enable new PD biomarkers to come to fruition by enabling access to harmonized longitudinal clinical and -omics data from over 4000 participants, including both individuals with PD and healthy controls. We also continue our robust collaboration with the National Institute on Aging to fund research in Alzheimer’s disease (AD) and Alzheimer’s disease-related dementias (ADRD). In March, NINDS hosted the triennial ADRD Summit to review and update priorities and timelines for addressing the ADRDs—including frontotemporal (FTD), Lewy body (LBD), mixed, and vascular dementias. Those updates will guide NIH investments in ADRD research, and we are grateful to the many stakeholders who have participated in the planning process.
Conducting the best science goes hand in hand with empowering the next generation of scientists, and so I am thrilled that NINDS recognizes outstanding mentors through the Landis Award for Outstanding Mentorship. In making the second year of awards this past summer, we want the community to know just how important mentorship is for sustaining the scientific research enterprise. To achieve our research and scientific goals, both at NINDS and at NIH as a whole, it truly is imperative that we value the efforts of outstanding mentors and lift up a diverse range of individuals to position them for success. Toward that end, I made a commitment earlier this year along with Francis Collins to only give presentations at conferences where diversity and inclusion are actively considered in panels or other prominent speaking slots, as diversity is not only important for representation, but also for making the science better.
As a step in making that science better, I also want to emphasize the utmost importance in maintaining healthy workplace climates, both here at NINDS and in the research environments that we support. Here at NINDS and at NIH, we are coming to grips with cultures and environments that have permitted unacceptable behavior for too long. To do better, we are taking action. Dr. Collins created a Working Group of the Advisory Committee to the NIH Director on Changing the Culture of Sexual Harassment, and NINDS is organizing a series of town halls early in 2020 that will be mandatory for all staff. There is no easy answer to addressing this issue, but it is critical that we work together to foster a culture of respect for all.
In 2019, NINDS supported innovative scientific advances in neuroscience and neurology. While I cannot highlight all of them here, I have selected a few favorites that you can view above. The past also brought us opportunities to explore and build new research programs, collaborations, and partnerships. All of our efforts at NINDS move forward (in no small part), thanks to our leadership team. In February, Dr. Lorna Role joined NINDS as Scientific Director, bringing her pioneering research and broad leadership experience to direct the intramural program (IRP), which includes 48 labs and 1000+ staff. Dr. Role has studied the brain’s cholinergic system over the lifespan, been the principal investigator on numerous NIH-funded grands, and earned numerous awards and honors including the NIH Director’s Pioneer Award. Under the guidance of strong NINDS leaders, we are poised for even more progress in 2020. In this new decade, which begins with a 7.8% increase in Congressionally appropriated funding over last year, we continue our important mission: seeking fundamental knowledge about the nervous system and using that knowledge to reduce the burden of neurological disease.










