A Breakthrough Beneath the Skin

It started with a debate over a poster.
At a medical conference in 2008, two neurologists—Dr. Christopher Gibbons and Dr. Roy Freeman—were deep in discussion. On the wall in front of them: a poster asking a provocative question about the presence of phosphorylated alpha-synuclein (p-Syn) in the gut.
But Freeman saw something more: a new window into the nervous system.
For decades, neurologists had noticed something curious. During autopsies, patients with Parkinson's disease and similar disorders showed distinctive damage to nerve cells, with abnormal accumulations of a protein called phosphorylated alpha-synuclein. The problem? These findings came too late—after patients had already endured years of uncertainty, misdiagnosis, and ineffective treatment.
What if we could detect this same protein—not postmortem, not in the brain—but in the skin?
This simple yet radical idea would take more than a decade to prove. But it would go on to become a revolutionary new diagnostic tool—The Syn-One Test—capable of identifying early signs of Parkinson's disease and related disorders through a simple skin biopsy.
And it all began with a spark of curiosity and critical NIH support.
From Lab Bench to Living Rooms

Between 2008 and 2020, the NIH's National Institute of Neurological Disorders and Stroke (NINDS) supported early foundational research at Beth Israel Deaconess Medical Center, led by Drs. Freeman and Gibbons, and at Vanderbilt University.
With funding from multiple NIH mechanisms—including K23 Mentored Patient-Oriented Research Career Development grants and a U54 Rare Diseases Clinical Research Consortia Award—Gibbons and Freeman began studying whether phosphorylated alpha-synuclein (a protein known to clump in the brains of Parkinson's patients) might also show up within cutaneous nerves embedded in the skin.
In 2013, they published their first findings. A year later, other scientists confirmed them. Still, they faced skepticism.
There were critics. People said it was ridiculous. Why would you find this in the skin?
Then something remarkable happened.
Doctors began reaching out, asking for help. They started sending skin samples from patients who were hard to diagnose—people who urgently needed answers.
The science wasn't just promising. It was working.
"Maybe we need to bring this to more people"

In 2017, at the American Academy of Neurology conference, Gibbons and Freeman met Dr. Todd Levine—a neurologist with entrepreneurial experience.
What you're doing is too important to stay in a lab.
And so began CND Life Sciences, a mission-driven company dedicated to bringing the Syn-One Test out of academia and into clinics across the country.
But they had a problem.
They had no lab. No money. No commercial infrastructure.
What they did have was a powerful idea—and soon, the support of an NIH Small Business Innovation Research (SBIR) grant.
The First Breakthrough: SBIR Funding

CND Life Sciences began in a tiny office in Phoenix, Arizona. Deliveries went to Dr. Todd Levine's house. There was no staff, just the founders building a lab from scratch. Then came the first SBIR Phase II award—a $3 million investment that changed everything.
That first SBIR grant didn't just fund our first real lab—it validated the entire technology.
With this support, they launched the Syn-One Study during the height of the COVID-19 pandemic. The funding allowed them to move forward when everything else was stalled. And just as importantly, it helped them raise private capital and gain credibility among clinicians.
We wouldn't be anywhere near where we are if we hadn't had that first SBIR grant.
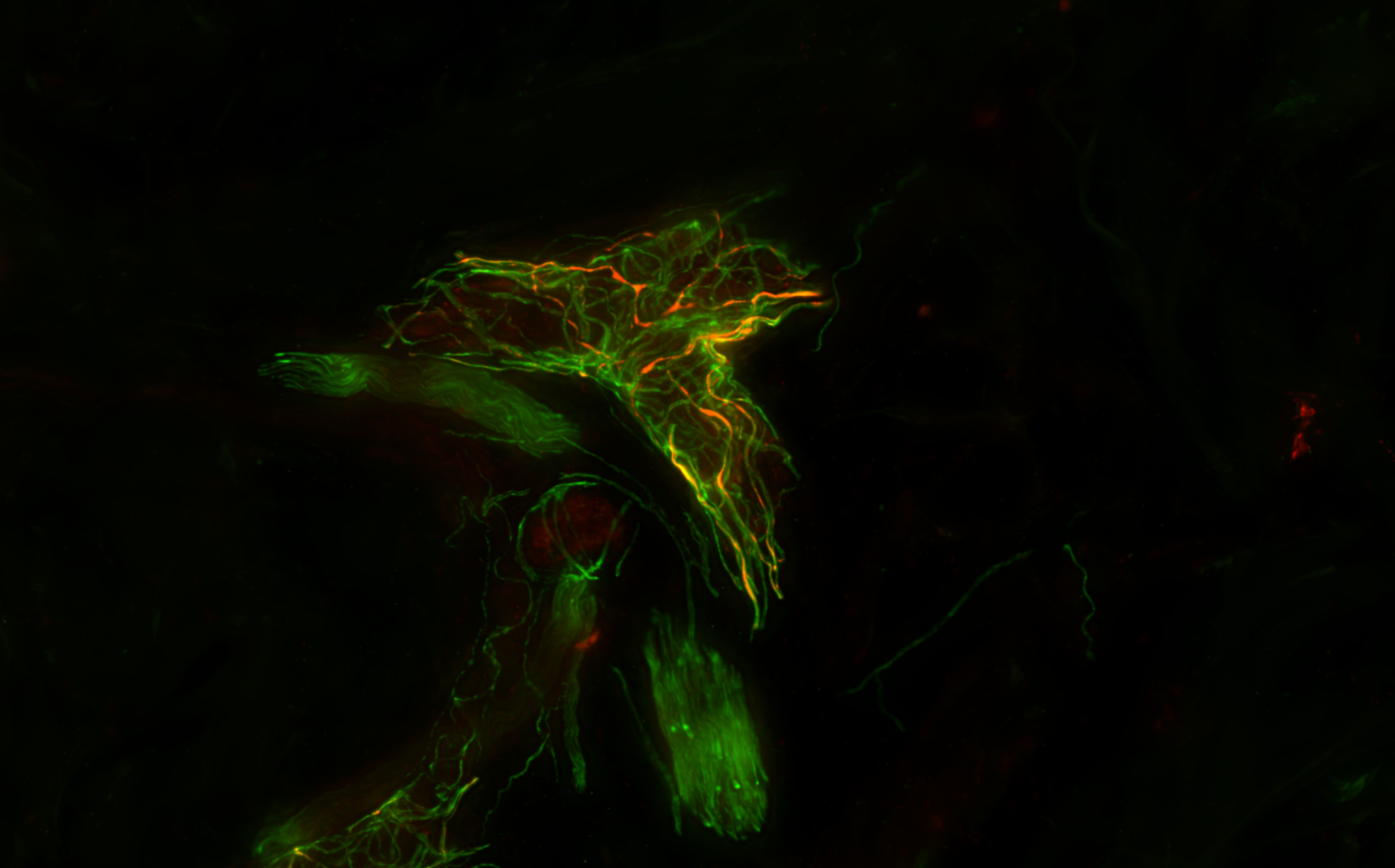
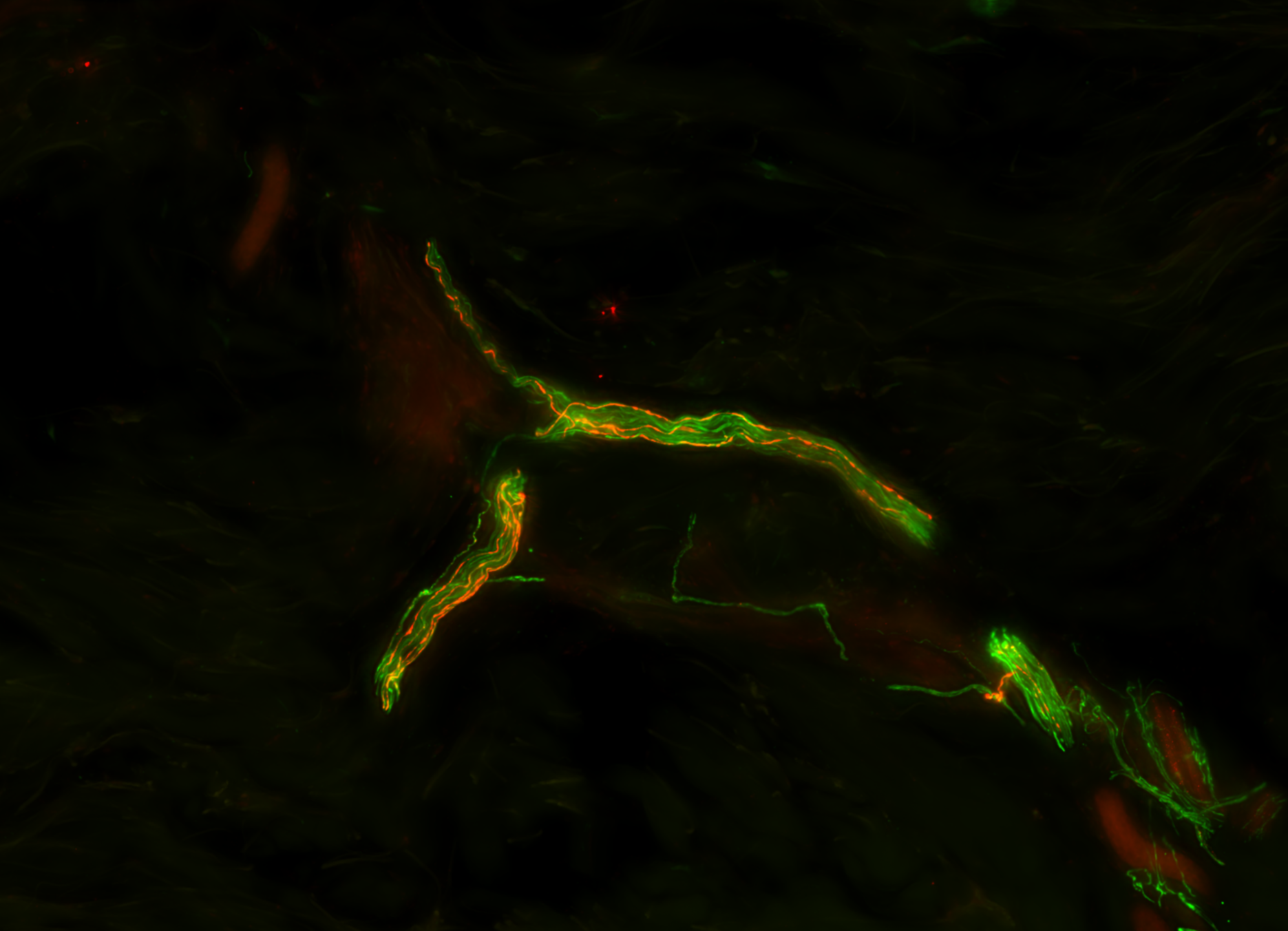
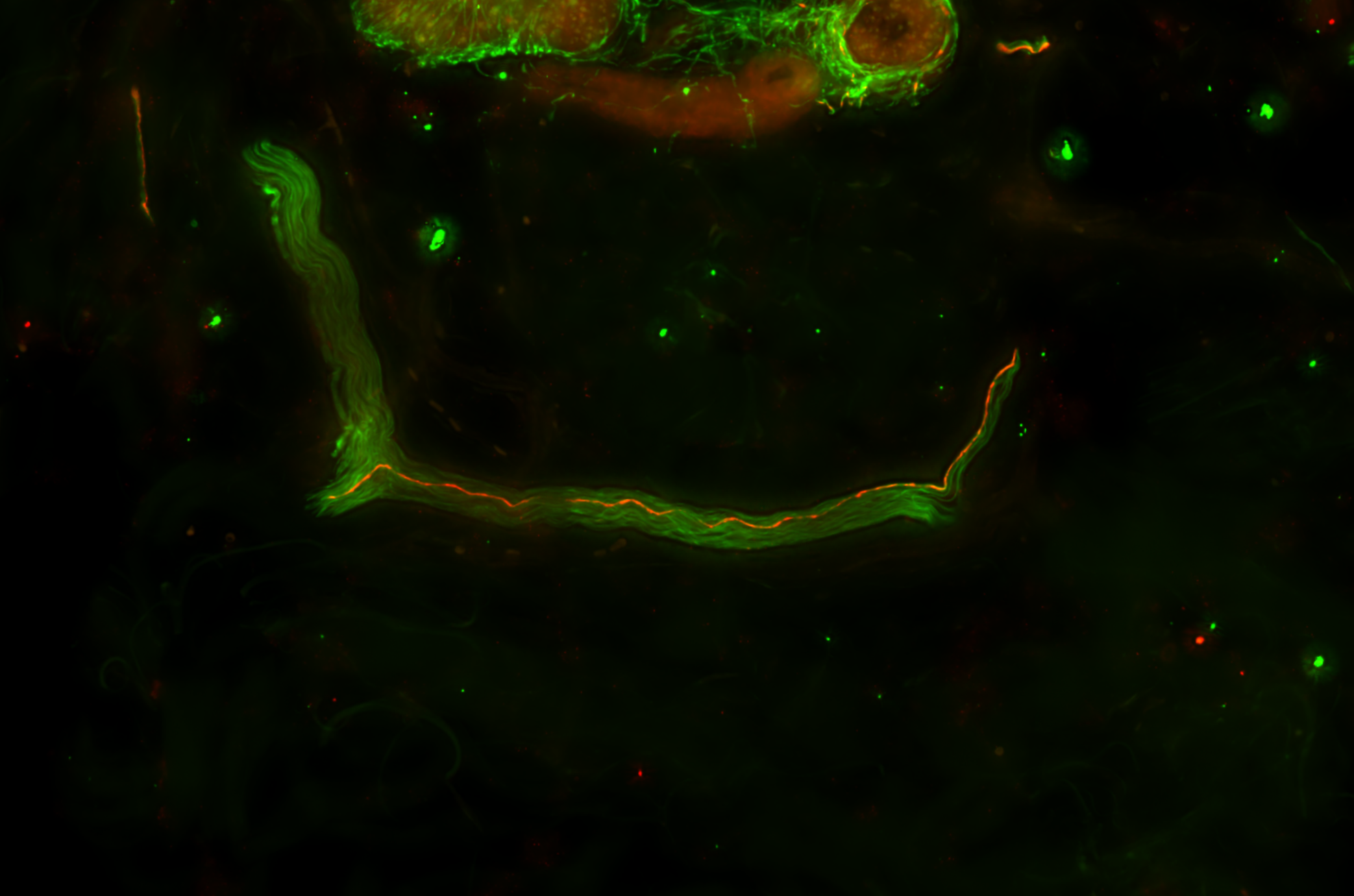
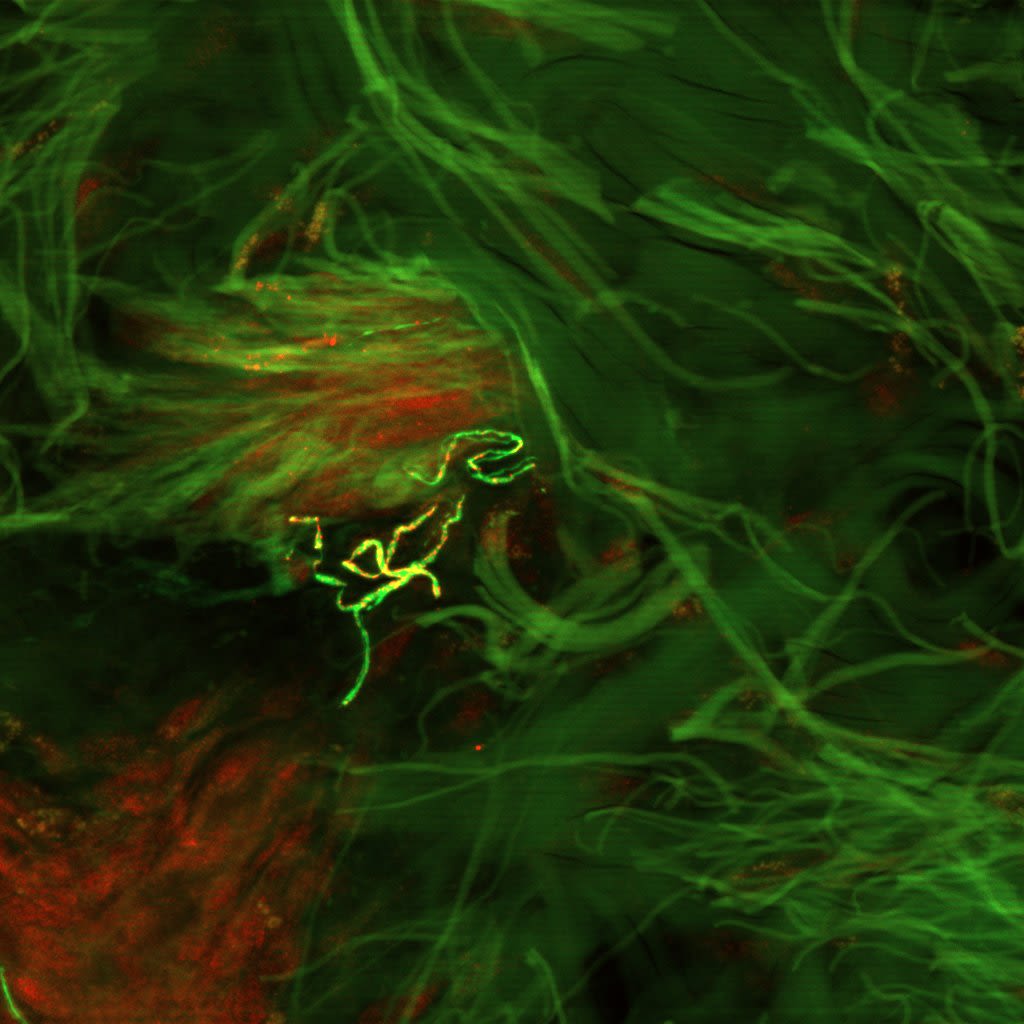
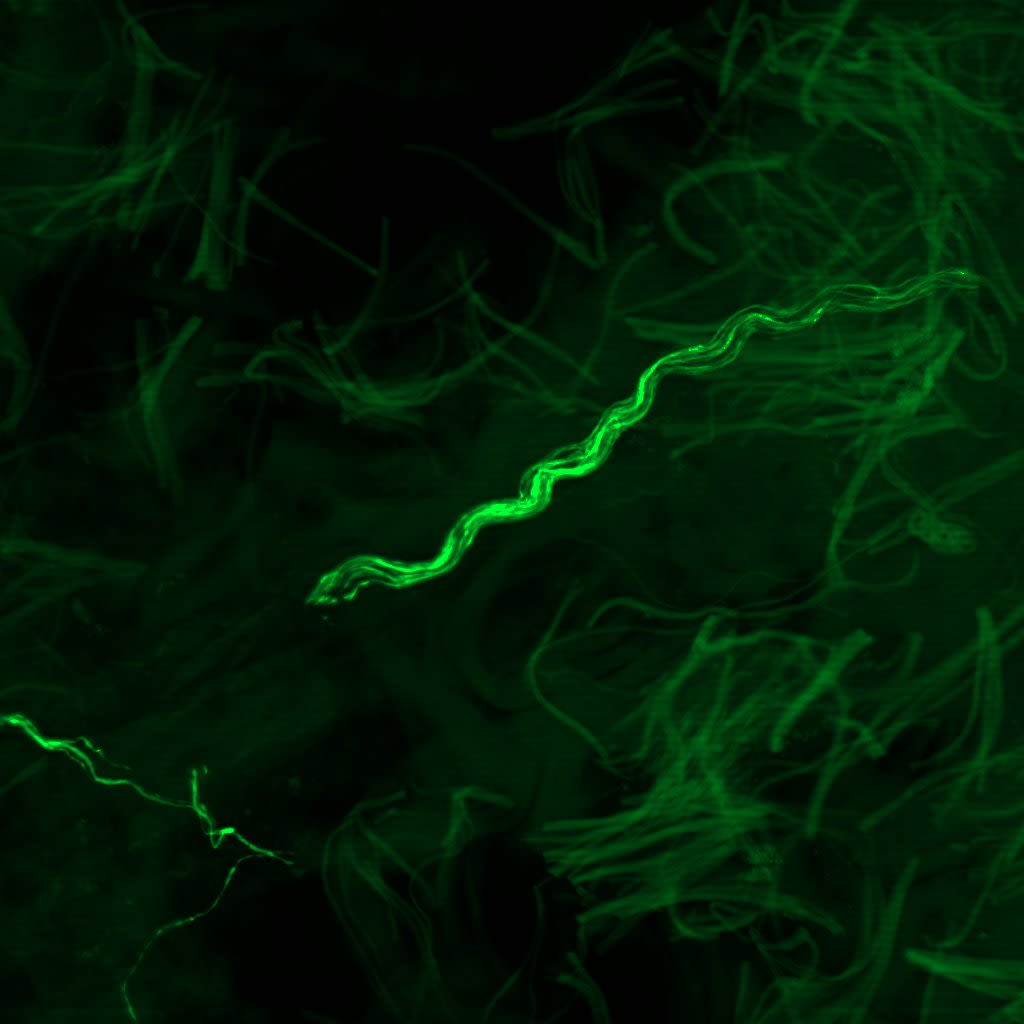
A Test That Changes Lives

The Syn-One Test is now helping doctors and patients across all 50 states. In just five years, it has guided diagnoses for more than 37,000 patients.
It is also democratizing access to neurological care. The test can be administered by primary care doctors—even in rural areas—offering insights once available only through invasive, difficult to access, or expensive procedures like brain imaging or spinal taps.
80% of people with Parkinson's will never see a movement disorder specialist. But now, a simple skin biopsy can help get them the answers they need.
The Science Marches Forward

Today, continued grants from the NIH and the Michael J. Fox Foundation have fostered the Syn-Q Study, the next evolution in skin-based diagnostics. Using AI, 3D imaging, and advanced pathology, researchers at CND are now quantifying alpha-synuclein levels and tracking how they change over time.
This could revolutionize how we evaluate treatments—not over years, but in weeks.
You can't defeat a disease if you can't measure it.
Why Continued Congressional Funding Matters

The Syn-One Test is just one example of what happens when NIH, academic scientists, private industry, and congressional support come together.
It was built across funding mechanisms—from K23 to U54, from SBIR to clinical trial support. Each one added a stepping stone across the "valley of death" where so many promising ideas are lost.
This type of discovery never happens without NIH. The SBIR program, in particular, helped us bridge the lab and the real world.
Investing in the Future of Brain Health

Skin-based diagnostics could unlock answers in diseases beyond Parkinson's— ALS, frontotemporal dementia, progressive supranuclear palsy, and more. These are conditions rooted in protein misfolding, and now we can search for their footprints beneath the skin.
Every grant, every partnership, every Congressional dollar invested has helped move these discoveries from theory to treatment.
One Last Word...
This all started with an idea that people told us was impossible. But thanks to NIH support, we turned that idea into a test that's helping thousands of patients get answers. Without that first grant, none of this would have happened.
Learn More
- NINDS Small Business Program
- Types of NINDS Small Business Grants
- Explore More at NINDS
- CND Life Sciences: A New Milestone in Diagnosing Parkinson's Disease and Related Disorders
We extend our appreciation to CND Life Sciences for sharing insights into the development of their Syn-One test and for providing the accompanying visuals. This research, supported by NINDS funding, represents important progress in advancing scientific discovery and diagnostic innovation.


