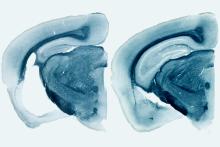
In a mouse model of dementia, the presence of ApoE4 produces significant degeneration in the brain (left) compared to mice without the gene (right). Image courtesy of Holtzman lab
NIH-funded study finds genetic risk factor interacts with tau protein
For more than 25 years, the protein ApoE4 has been linked to late onset Alzheimer’s disease (AD). ApoE has also been linked to the formation of amyloid-β deposits in the brain, one of the hallmarks of Alzheimer’s disease (AD). Now researchers have connected ApoE4 to the toxic accumulation of another protein, tau, suggesting a possible new target for therapy. This work was published in Nature and supported by the National Institutes of Health (NIH).
“If ApoE4 can accelerate tau-dependent neurodegeneration without involving amyloid-β, it could be a real game-changer in the way we think about these proteins and their link to dementia,” said Roderick A. Corriveau, Ph.D., program director at the National Institute of Neurological Disorders and Stroke (NINDS), a part of the NIH.
Tau is a protein that plays an important role in maintaining structure of neurons. In several different forms of dementia known as tauopathies, the protein can aggregate into clumps of varying sizes that lead to tangle formation and eventually nerve cell degeneration.
In this study, a team led by David M. Holtzman, M.D., professor and chair of the Department of Neurology, Washington University School of Medicine and senior author of the study, showed that the presence of ApoE4 can increase the amount of damage due to tau aggregates, while a lack of any ApoE protein protects the brain from tau-induced damage.
“We knew that the accumulation of tau was important for cognitive decline and that ApoE4 is a risk factor for developing Alzheimer’s disease, but this is the first direct evidence of a link between the two,” said Dr. Holtzman.
ApoE is a protein that is involved in transporting cholesterol throughout the body and comes in three different forms in humans: ApoE2, 3, and 4. Compared to people with the most common type, ApoE3, individuals with ApoE4 are at an increased risk of developing AD, while ApoE2 appears to offer some protection against the disease.
Dr. Holtzman and his group studied the effects of ApoE in a mouse model of tauopathy that overproduces human tau by replacing the normal mouse ApoE gene with either human ApoE2, 3, or 4. At 9 months of age, the mice with the ApoE4 gene showed significant damage in the brain, with considerable shrinkage in the hippocampus, an area critical for memory. Mice that had no ApoE gene showed almost no degeneration.
Taking a closer look, Dr. Holtzman and his colleagues saw increased inflammation in the brain and the activation of immune cells called microglia, particularly in mice with the ApoE4 gene. This activation caused astrocytes, another type of cell in the brain, to turn on genes associated with immune responses and inflammation. Dr. Holtzman’s team further studied this effect by growing astrocytes from mice that either made ApoE4 or were missing the ApoE gene entirely in dishes along with tau-containing neurons. The ApoE4-containing dishes had significant neuronal death while the dishes without ApoE did not.
“The combination of ApoE4 and tau is clearly producing a strong inflammatory response that leads to significant cell death in the brain,” said Dr. Holtzman. “In contrast, tau accumulation has little effect in the complete absence of ApoE protein.”
Alzheimer’s disease is the most common form of tauopathy, affecting as many as 5.3 million Americans age 65 and older. The ApoE4 gene has long been associated with increased risk for AD and the accumulation of amyloid-β in the brain. However, research has shown that amyloid-β aggregates alone do not produce the full spectrum of symptoms of AD nor do they lead to extensive brain cell death, prompting researchers to look for additional roles for ApoE4 in exacerbating the disease.
Most therapies being studied for AD have focused on eliminating the accumulation of either amyloid-β deposits or tau tangles. This study suggests that efforts to reduce ApoE in the brain could have potential as a new treatment.
“There are people out there who have no gene for ApoE, and they seem to be just fine cognitively,” said Dr. Holtzman. “Because ApoE doesn’t seem to be required for normal brain function, reducing it in the brains of early stage Alzheimer’s disease patients could be a viable treatment, but this has not yet been tested.”
In addition to testing ways of targeting ApoE using gene therapy methods, Dr. Holtzman and his colleagues are also hoping to better understand the mechanism by which ApoE interacts with tau to cause cell death in the brain.
“This area of research, along with other studies on the connection between ApoE4 and tau, opens the door to a whole new understanding of the interplay between these two molecules and Alzheimer’s,” said Eliezer Masliah, M.D., director, Division of Neuroscience, National Institute on Aging (NIA), a part of the NIH. “Along with the entire Alzheimer’s research community, we look forward to future work exploring the many possibilities revealed by this connection.”
The study was funded by the NINDS (NS090934, NS088137, NS096719-01), the NIA (AG03991, AG026276, AG05681, AG023501, AG019724, AG052648, AG051812), the JPB Foundation, Cure Alzheimer’s Fund, AstraZeneca, Consortium for Frontotemporal Dementia Research, Tau Consortium, National Multiple Sclerosis Society, Nancy Davis Foundation, and Amyotrophic Lateral Sclerosis Association.
Article:
Shi et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature, September 20, 2017. DOI: 10.1038/nature24016
For more information:
https://www.ninds.nih.gov/Disorders/All-Disorders/Alzheimers-Disease-Information-Page
https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Hope-Through-Research/Dementia-Hope-Through-Research
http://www.ninds.nih.gov
https://www.nia.nih.gov/health/alzheimers-disease-fact-sheet
https://www.nia.nih.gov/health/video-how-alzheimers-changes-brain
https://www.nia.nih.gov/health/causes-frontotemporal-disorders
###
The NINDS (http://www.ninds.nih.gov) is the nation’s leading funder of research on the brain and nervous system. The mission of NINDS is to seek fundamental knowledge about the brain and nervous system and to use that knowledge to reduce the burden of neurological disease.
About the National Institute on Aging: The NIA leads the federal government effort conducting and supporting research on aging and the health and well-being of older people. It provides information on age-related cognitive change and neurodegenerative disease specifically at its Alzheimer's Disease Education and Referral (ADEAR) Center at www.nia.nih.gov/alzheimers. For additional information about cognitive health and older adults, go to NIA’s cognitive aging portal at www.nia.nih.gov/health/cognitive-health.
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit http://www.nih.gov.
