Genome editing, which enables precise modifications to DNA, has the potential to lead to breakthrough treatments for diseases caused by genetic changes. In 2018, the National Institutes of Health (NIH) launched the Somatic Cell Genome Editing (SCGE) program to remove barriers to the clinical adoption of genome editing techniques to treat a variety of disorders. Funded through the NIH Common Fund, which supports NIH-wide programs that are designed to be short-term and to address emerging scientific opportunities, SCGE is now entering its second phase with a series of new awards.
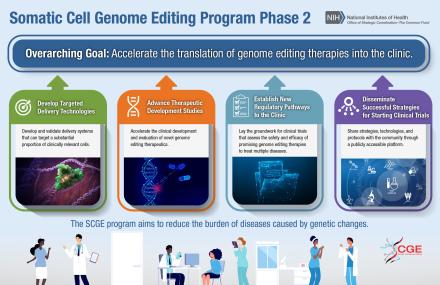
Specifically, Phase 2 of SCGE will focus on the following four areas:
- Development of new technologies and assays to aid in developing gene-editing treatments that can be submitted to the Food and Drug Administration (FDA) for approval.
- Studies of promising somatic genome editing candidates that could lead to FDA submissions.
- Planning for platform clinical trials to develop somatic genome editing approaches for multiple diseases.
- Establishment of a Translational Coordination and Development Center for SCGE.
The newly funded projects cover three of these areas, with awards related to platform clinical trials to be announced later this summer. All build upon the work performed though the SCGE over the past five years and will focus on translating safe and effective genome editing therapeutics into the clinic.
"Genetic mutations can cause some of the most rare and devastating disorders of the nervous system," said Walter Koroshetz, M.D., director of the National Institute of Neurological Disorders and Stroke (NINDS) and co-chair of SCGE. “Thanks to large-scale efforts like the Somatic Cell Genome Editing Program, we are starting to bring tools into the clinic to edit out these gene mutations. While there are still challenges to overcome, the level of hope for effective treatments is high.”
The genome comprises a person’s complete DNA sequence, and many diseases have been linked to changes in DNA, i.e., mutations, that can be either inherited from one or both parents or accumulated during a person’s lifetime. Somatic cells are those cells that make up the various organs of the body and are not part of the reproductive process, which means that edits to somatic cells only affect treated patients and cannot be passed on to future generations. The ability to edit the DNA in somatic cells means that tissues or organs affected by a specific mutation could have that mutation corrected in a way that either restores lost function; prevents further degeneration within that tissue; or otherwise slows or halts the progression of disease.
“Genome editing is a promising technology that could lead to treatments for many rare diseases,” said Joni L. Rutter, Ph.D., National Center for Advancing Translational Sciences (NCATS) director and SCGE co-chair. “The SCGE program has advanced the field, and these new funding awards will help researchers take the next step toward turning laboratory discoveries into potentially life-saving therapies for the patients who need them.”
Several Phase 1 results were included along with reviews of some of the most promising genome editing techniques in a February 2022 collection of articles published in the Nature portfolio of journals. These included editing hematopoetic (blood) stem cells in mice to rescue sickle cell disease; the successful modification of a gene target that extends the lifespan of a mouse model of progeria, a disease that causes extremely rapid aging; and a number of enhanced delivery methods and technologies designed to more precisely target specific tissues and cell types and to improve the genome editing process.
Phase 2 of SCGE is funded by the Common Fund and jointly led by NINDS and NCATS.
New Awards
The newly funded awards expand the scope of SCGE by addressing barriers to moving therapies from lab to the clinic. For instance, one project aims to use human organoids, which are derived from induced pluripotent stem cells—created from a patient tissue sample, typically from skin or blood—to create “mini organs” representing kidney, liver, lung, brain, retina, and/or the heart in dishes. This technology would allow editing techniques to be tested safely in lab-grown tissues/organs possessing the human genome.
Another project takes aim specifically at four rare neurological diseases—spinal muscular atrophy, Friedrich’s ataxia, Huntington’s disease, and Rett syndrome—that represent a significant unmet clinical need that could be addressed through genome editing therapy. A third project will develop a method for testing the potency of genome editing-based treatments for sickle-cell disease, which affects roughly 100,000 individuals in the United States and millions worldwide.
Another key component to Phase 2 of SCGE is the establishment of a Translational Coordination and Dissemination Center (TCDC). By leading consortium-wide activities, the TCDC will help facilitate collaborations among SCGE researchers. The TCDC will also be responsible for developing the means for data and knowledge sharing across the consortium and providing a publicly available online platforms for maintaining and sharing the data and insights gathered in both phases of SCGE.
In addition to NINDS and NCATS, other NIH Institutes and Centers managing these awards include the National Institute of Allergy and Infectious Diseases (NIAID) and National Heart, Lung, and Blood Institute (NHLBI).
To futher support the SCGE’s commitment to developing targeted delivery systems for genome editing, the NIH also recently announced the TARGETED (Targeted Genome Editor Delivery) Challenge. This first phase of this multi-phase challenge is open to all eligible competitors. Learn more about the TARGETED Challenge.
Learn more about somatic cell genome editing; program grantees and award information; and the 2018 and 2019 award announcements from Phase 1.
