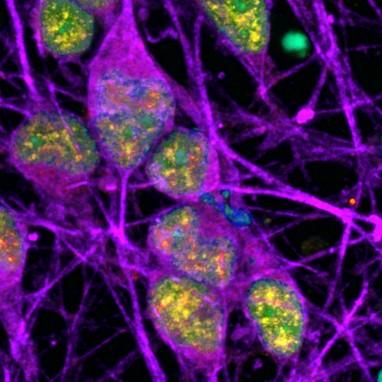
Researchers at the National Institutes of Health (NIH) have discovered specific regions within the DNA of neurons that accumulate a certain type of damage (called single-strand breaks or SSBs). This accumulation of SSBs appears to be unique to neurons, and it challenges what is generally understood about the cause of DNA damage and its potential implications in neurodegenerative diseases. In this study, damage within neurons was often found within specific regions of DNA called “enhancers” that control the activity of nearby genes. Interestingly, a significant number of SSBs occurred when methyl groups were removed (which typically make a gene available for activation), suggesting that removal of methyl groups is linked to SSBs and that DNA damage may be part of the normal process of switching genes on and off. Furthermore, it implies that defects in the repair process, not the DNA damage itself, can potentially lead to developmental or neurodegenerative diseases.
Image: Neurons (labeled in purple) show signs of an active DNA repair process (labeled in yellow). The cells’ DNA itself is labeled in cyan (in this image, overlap between cyan and yellow appears green).
Article: Wu W. et al. Neuronal enhancers are hot spots for DNA single-strand break repair. March 25, 2021. Nature.
NIH Press Release: DNA damage “hot spots” discovered within neurons

NIH BRAIN Initiative-funded scientists have developed a brain-computer interface (BCI) designed to restore the ability to communicate in people with spinal cord injuries and neurological disorders such as amyotrophic lateral sclerosis (ALS). This system has the potential to work more quickly than previous BCIs, and it does so by tapping into one of the oldest means of communications we have—handwriting. The researchers focused on the part of the brain that is responsible for fine movement and recorded the signals generated when their participant attempted to write individual letters by hand. In doing so, the participant, who is paralyzed from the neck down following a spinal cord injury, trained a machine learning computer algorithm to identify neural patterns representing individual letters. While demonstrated as a proof of concept in one patient so far, this system appears to be more accurate and more efficient than existing communication BCIs and could help people with paralysis rapidly type without needing to use their hands.
Image: Two implanted electrode arrays record the brain activity produced by thinking about writing letters. This information is then collected and processed in real-time by a computer, which converts that data into words on a screen.
Article: Willett FR et al., “High-performance brain-to-text communication via handwriting.” Nature. May 12, 2021.
NIH Press Release: Composing thoughts: mental handwriting produces brain activity that can be turned into text

By studying lab-grown neurons derived the skin or blood cells of patients with ALS causing mutations, as well as patients with non-inherited ALS, researchers have found a possible starting point for the dysfunction that causes the disease. They report that there is an accumulation of a protein called CHMP7 in the nucleus of ALS cultured nerve cells, as well as in ALS samples from the brain region that controls movement. Treatments that decreased the amount of CHMP7 in the cultured cells prevented a series of abnormalities that are characteristic of ALS, providing a possible therapeutic target for the disease.
Image: When CHMP7 accumulates in the nucleus, certain proteins become missing from nuclear pores (outlined in white). This causes the pores to break apart, leading to downstream effects that may cause ALS.
Article: Coyne A.N. et al. Nuclear accumulation of CHMP7 initiates nuclear pore complex injury and subsequent TDP-43 dysfunction in sporadic and familial ALS. Science Translational Medicine. July 28, 2021.
NIH Press Release: Researchers identify a cellular defect common to familial and sporadic forms of ALS
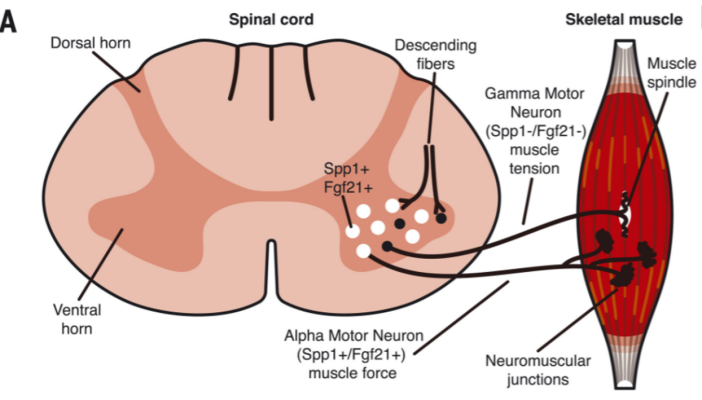
Researchers have identified a pathway common to several types of axonal peripheral neuropathies (APNs), including multiple forms of Charcot-Marie-Tooth (CMT) disease, and have identified a possible drug target that could help treat the disorder. Using a mouse model, the researchers looked at APN mice that were missing GCN2, a protein previously implicated in activation of the integrated stress response and defects in protein translation. These mice began to develop symptoms of the disease around two weeks of age, but the disease did not progress much beyond the initial stages. When the APN mice were instead treated with a drug to stop GCN2 from working, they showed improvements in many symptoms.
Image: Motor neurons in the ventral horn of the spinal cord include alpha (Spp1-positive) and gamma (Spp1-negative) populations, but only Spp1-positive cells express Fgf21 in mutant mice.
Article: Spaulding E.L. et al. The integrated stress response contributes to tRNA synthetase-associated peripheral neuropathy. Sept. 3, 2021. Science.
NIH Press Release: NIH-funded mouse study shows blocking stress response could slow axonal peripheral neuropathies disease progression

The NIH Brain Research Through Advancing Innovative Neurotechnologies® (BRAIN) Initiative Cell Census Network (BICCN) has unveiled an atlas of cell types and an anatomical neuronal wiring diagram for the mammalian primary motor cortex, derived from detailed studies of mice, monkeys, and humans. This publicly available resource represents the culmination of an international collaborative effort by more than 250 scientists at more than 45 institutions across 3 continents. The atlas provides a foundation for future research efforts that dive deeper into the structure and function of cells in the mammalian brain. These studies may include efforts to understand how the brain matures and develops, as well as research examining the roles that distinct cell types play in the creation of complex thought and behavior.
Image: A multicolor visualization of the mouse MOp transcriptomic taxonomy overlaid with mapped neuronal cells surrounded by color-coordinated pictures of different types of neurons (GABAergic, glutamatergic, chandelier, etc.) and their electrophysiological signatures.
Article: BRAIN Initiative Cell Census Network (BICCN). (2021). A multimodal cell census and atlas of the mammalian primary motor cortex. Nature.
NIH Press Release: NIH BRAIN Initiative Unveils Detailed Atlas of the Mammalian Primary Motor Cortex
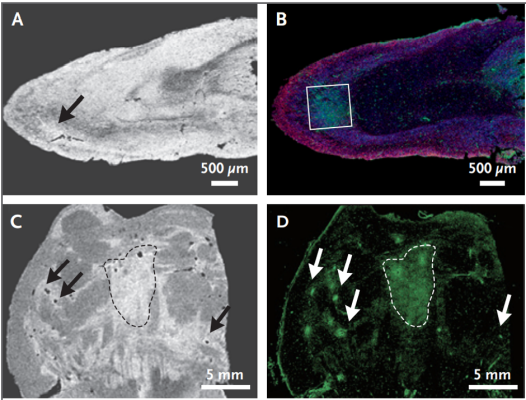
In a postmortem study, researchers identified abnormalities in brains of patients who died from Covid-19. The researchers performed magnetic resonance microscopy, histopathological evaluation, and immunohistochemical analysis on sections of the brain and the olfactory bulb. From this sample, they observed multifocal microvascular injury and fibrinogen leakage in both the brain and the olfactory bulb.
Image: Magnetic resonance microscopy of the olfactory bulb (top left) shows an area of hyperintense signal (arrow). Corresponding area on multiplex immunofluorescence imaging (top right), which revealed a focal area of fibrinogen leakage (in the box, fibrinogen is shown in green, collagen IV is shown in yellow, and nuclei are shown in blue). Magnetic resonance microscopy of the pons (bottom left), and fibrinogen staining (bottom right) shows areas of increased signal intensity corresponding to the vascular leakage (arrows and area in dashed lines) visible on magnetic resonance microscopy.
Article: Lee MH et al. Microvascular injury in the brains of patients with Covid-19. Feb 4, 2021. NEJM.
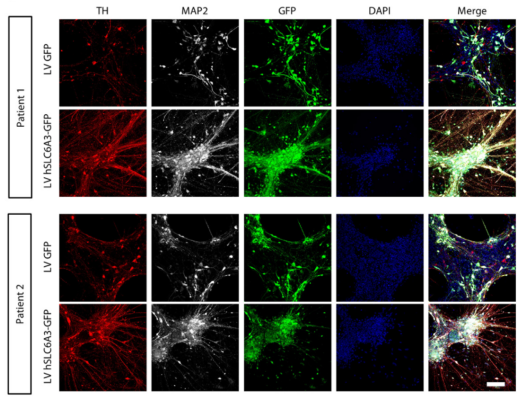
Researchers developed a gene therapy for dopamine transporter deficiency syndrome (DTDS), a rare neurodegenerative disease caused by harmful mutations in SLC6A3, which encodes the dopamine transporter (DAT). DAT is a crucial component in neurons in the brain that controls the levels of dopamine: disruption of this dopamine control in DTDS results in a progressive movement disorder in patients. This study found that delivery of human SLC6A3 improved the activity of DAT and reduced neurodegeneration in patient derived neurons. Further, the study found that injection of SLC6A3 in a region of brain called the midbrain of adult DTDS mice had therapeutic effects. This study brings promise for the treatment of other inherited neurodegenerative disorders, most of which are incurable.
Image: Immunofluorescence analysis at day 65 for patient-derived dopaminergic neurons transduced with LV GFP or LV hSLC6A3-GFP. Cells are stained for tyrosine hydroxylase (TH) and microtuble-associated protein 2 (MAP2), and nuclei were counterstained with DAPI. Scale bar, 100 μm.
Article: Ng J. et al. Gene therapy restores dopamine transporter expression and ameliorates pathology in iPSC and mouse models of infantile parkinsonism. May 19, 2021. Science.
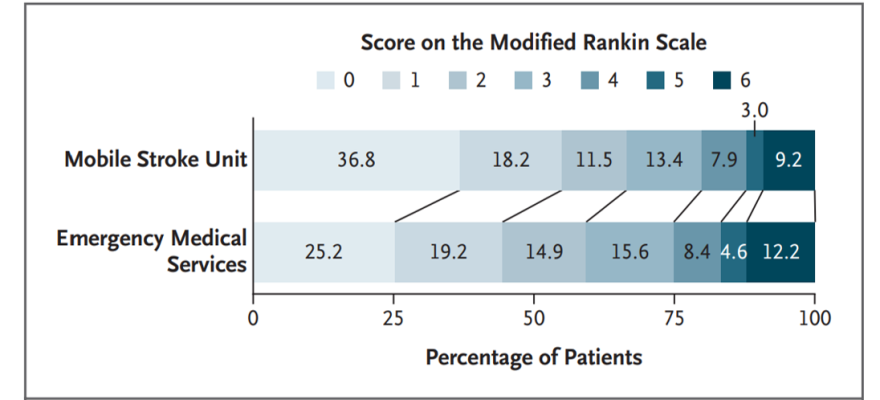
Stroke treatment – including administration of tissue plasminogen activator (t-PA) or endovascular thrombectomy (EVT) – is most effective as soon as possible after the onset of stroke, especially if t-PA can be delivered within the first hour after stroke onset. Mobile stroke units (MSUs) are ambulances with staff and a computed tomographic scanner, and they may be equipped to enable faster treatment with t-PA than standard management by emergency medical services (EMS). A recent prospective, multicenter, alternating-week, cluster-controlled trial compared clinical outcomes in patients eligible for t-PA who received care from a MSU as compared with standard care through EMS. Patients who received emergency care within 4.5 hours after stroke onset had less disability on a utility-weighted scale at 90 days with MSU management than with management by EMS.
Image: Distribution of scores on the modified Rankin scale (a measure of disability) at 90 days after tissue plasminogen activator (t-PA) administration shows that patients receiving emergency care with mobile stroke unit (MSU) management had lower disability scores relative to patients receiving care through emergency medical services (EMS).
Article: Grotta JC et al., Prospective, Multicenter, Controlled Trial of Mobile Stroke Units, September 9, 2021, The New England Journal of Medicine
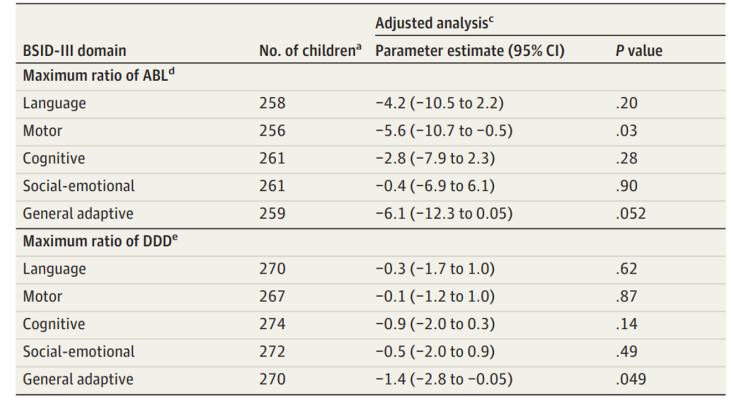
New findings published in JAMA Neurology suggest there is no difference in cognitive outcomes at age 2 among children of healthy women and children of women with epilepsy who took antiseizure medication during pregnancy. The findings are part of the large research project Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD), which is a prospective, long-term study looking at outcomes in pregnant women with epilepsy and their children. The study found that children born to mothers with the very highest levels of antiseizure medication in the blood during the third trimester had somewhat lower scores on tests in the motor and general adaptive domains, which refer to skills related to self-care, such as feeding. The children in this study will continue to be followed and will participate in additional cognitive tests through age 6. Results so far indicate that controlling epilepsy with these medications during pregnancy may be safe for babies.
Image: Ratio measurements of antiseizure medication (ASM) blood level (ABL) and defined daily dose (DDD) of ASM demonstrate no neurodevelopmental differences in children of healthy women and children of women with epilepsy up to age 2.
Article: Meador KJ et al., Two-year old cognitive outcomes in children of pregnant women with epilepsy in the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs Study, June 7, 2021, JAMA Neurology
NIH Press Release: Study suggests no association between antiseizure drugs used during pregnancy and neurodevelopmental problems in children at age 2
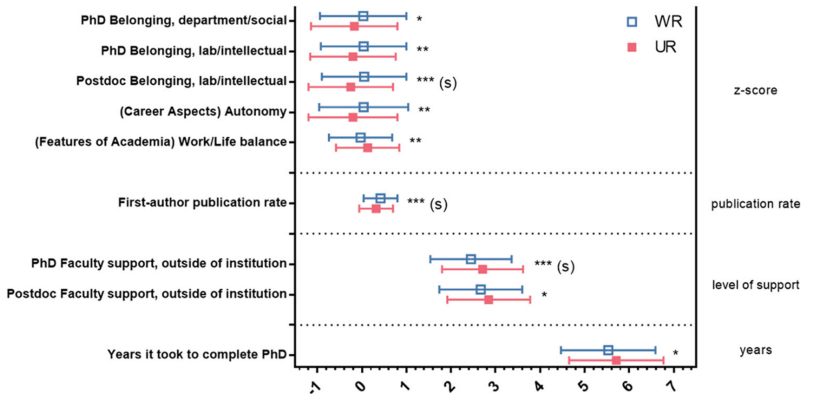
National Institutes of Health (NIH) officials surveyed 1,479 neuroscientists who had recently obtained a doctorate about their career views. As with earlier studies, this one found that interest in pursuing a career in academic research dropped as the scientists went through training. This was especially true for women and members of historically underrepresented racial and ethnic groups and it appeared that several factors, including lifestyle considerations and a sense of alienation, contributed to this trend. The authors concluded that these factors represent strategic challenges that both NIH policy makers and institutional grantees can address to enhance diversity in neuroscience. Results underscore the needs of women and members of underrepresented groups when training the next generation of neuroscientists.
Image: For under-represented (UR) and well-represented (WR) survey respondents, preferences about careers in general, and academic careers specifically, affected career interest and were moderated by social identity and experiences in graduate and postdoctoral training.
Article: Ullrich, L.E., Ogawa, J.R., & Jones-London, M.D. Factors that Influence Career Choice Among Different Populations of Neuroscience Trainees. May 26, 2021, eNeuro.
NIH Press Release: NIH study highlights systemic diversity issues in the neuroscience research community
With each new year comes possibility and promise. The challenges we have faced in 2021 continue to test our grit and in some ways, our patience. My deepest sympathies go out to those of you who have experienced loss associated with the COVID-19 pandemic. Yet despite these challenges, I have witnessed our research and scientific community endure and persevere. With this in mind, I am steadfast and certain that there is much to be hopeful for in 2022.
We are learning more about the effects of SARS-CoV-2 infection on the nervous system—in some cases, detrimental effects last far longer than the infection. NINDS is one of several Institutes, Centers, and Offices contributing to the NIH Researching COVID to Enhance Recovery (RECOVER) Initiative. The RECOVER Initiative seeks to understand the scope of the problem and how SARS-CoV-2 can lead to long-lasting and widespread effects such as fatigue, decline in some cognitive abilities, pain, sleep disorders, and dysautonomia, among others. In September, NIH awarded nearly $470 million to build a national study population of diverse research volunteers and support large-scale studies on the long-term effects of COVID-19. I am proud of NINDS contributions to this collective effort.
NINDS meetings, conferences, and workshops have continued virtually in 2021. While these virtual venues are no replacement for the valued in-person interactions at scientific conferences, we have leveraged the ability of a virtual meeting to reach many more people than we normally would, including at this year’s NINDS Nonprofit Forum and 7th Annual BRAIN Initiative Meeting. These virtual gatherings have also been valuable in soliciting community input and collecting expert perspectives, such as through our Health Disparities and Inequities in Neurological Disorders Workshop (HEADWAY). The upcoming year will give us additional virtual gathering opportunities, including the Alzheimer's Disease-Related Dementias Virtual Summit. We must continue to plan for virtual meetings until we can gather safely in person, and I want to take a moment to recognize the active participation from the scientific community and the many NINDS staff who have worked tirelessly to ensure that virtual meetings function as seamlessly as possible.
This past year has also led to much personal reflection on the NINDS mission statement of generating knowledge to reduce the burden of neurological disorders, for all people. I am encouraged that the events of 2020 have catalyzed numerous NIH efforts to improve workforce diversity, including the UNITE Initiative to identify and address structural racism within the NIH-supported and greater scientific community, the Plan for Enhancing Diverse Perspectives through the NIH BRAIN Initiative, and the Faculty Institutional Recruitment for Sustainable Transformation (FIRST) program, which aims to enhance and maintain cultures of inclusive excellence in the biomedical research community. NINDS has contributed effective and creative programs on workforce diversity for years; in addition, this year, we reported on factors that influence career choices among different populations of neuroscience trainees, and on understanding disparities in career equity. Ensuring a vibrant, talented, and diverse community of neuroscientists is also one of the cross-cutting strategies outlined in our 2021-2026 NINDS Strategic Plan, which covers both the extramural, intramural and NIH workforce.
As we continue with online meetings and collaboration spaces with an eye towards safe in-person gatherings in 2022, I am proud of NINDS contributions to trans-NIH Initiatives. These have included NINDS participation to better understand amyotrophic lateral sclerosis (ALS) through the Accelerating Leading-edge Science in ALS (ALS2) initiative, an effort to rapidly advance our understanding of what triggers ALS and what drives its rapid progression. In large part enabled by Congress’s funding of the National Action Plan for Alzheimer’s Disease, NINDS has led ambitious new research on Alzheimer's Disease and Alzheimer's Disease Related Dementias (AD/ADRD). In addition, in recognition of the urgent need to improve the management of pain, NINDS has also contributed to trans-NIH pain research efforts and opportunities including through the Helping to End Addiction Long-term® Initiative, or NIH HEAL Initiative®. Finally, NINDS also plays a critical role in the truly amazing NIH BRAIN Initiative, which published a series of landmark papers detailing the cell types, their connections and physiological properties in motor cortex of mouse, non-human primate and human.
In 2021, NINDS continued to support investigators who – even in the midst of a pandemic – persevered with numerous novel innovative scientific advances in neuroscience and neurology. While I cannot highlight all of them here, I have selected a few favorites that you can view above. The past also brought us opportunities to explore and build new research programs, collaborations, and partnerships. All of our efforts at NINDS move forward (in no small part), thanks to our leadership team. In January, I named Dr. Amy Bany Adams as Deputy Director for Scientific Management and Operations, a position established to accelerate the pace of neuroscience research by strengthening the partnerships between science and administration. In addition, in March, NIH announced Dr. Andrea Beckel-Mitchener as deputy director of the NIH BRAIN Initiative. Alongside BRAIN Director Dr. John Ngai, she is working with NIH staff and scientists around the world, helping to oversee and coordinate research opportunities to develop new technologies for examining cell and circuit function in the brain.
Each year also brings transitions: after two years, Dr. Lorna Role stepped down in her role as NINDS Scientific Director to return to her lab as a full-time scientist. While this is a definite loss for NINDS, I am delighted that Lorna will remain a part of our scientific community, contributing to the understanding of mechanisms underlying neurodegenerative disorders with a focus on cholinergic circuits. I also want to acknowledge NINDS Deputy Director Dr. Nina Schor who has graciously stepped in to serve as acting Scientific Director with incredible effectiveness. Finally, I am also grateful for the leadership, tenacity, and friendship of NIH Director Dr. Francis Collins, who stepped down from his position after a 12-year tenure. Moving into 2022, we look forward to a new NINDS Scientific Director and NIH Director, whose visions and leadership will shepherd us towards an even more productive year.
With determination and hope, I am excited for all that NINDS and our community can bring to scientific progress in 2022, both in our ability to reduce the effects of the COVID-19 pandemic and in our research priorities as we implement our new 2021-2026 NINDS Strategic Plan. To that end, we continue to await Congressionally-approved appropriations, and as of this writing, NIH is still operating under a Continuing Resolution, i.e., guided by legislative mandates that were in effect in Fiscal Year 2021, until a final budget for Fiscal Year 2022 is approved by Congress.
Through it all, we will continue our important mission: seeking fundamental knowledge about the nervous system and using that knowledge to reduce the burden of neurological disease, for all people.










