The beginning of a new year has a way of sneaking up on all of us, but we always gain helpful perspectives by stopping to reflect on the year that has passed. In 2017, NINDS made significant strides through funding awards, collaboration, new research initiatives, and leadership additions. We are grateful to our investigators, research subjects, and our partners representing those suffering from neurological disorders and stroke.
In 2017, NIH launched an Opioid Initiative to marshal our resources to address the public health crisis of opioid addiction, misuse and overdose. In June and July, the NIH hosted meetings, led by NIH Director Dr. Francis Collins, that brought together experts from government, industry, and academia. Emerging from those discussions, NIH is working with FDA and private sector experts to develop a public-private partnership to advance non-addictive, pharmacological treatments for pain and more effective ways to combat addiction and overdose deaths. NINDS leads the NIH Pain Consortium made up of Institutes and Centers all working to advance our knowledge of the biology of pain and find safe and effective treatments. In addition, NINDS led the development of the Federal Pain Research Strategy, a long-term strategic plan to advance the federal pain research agenda, which was released in September. Finally, in conjunction with the Office of the Assistant Secretary for Health in the Department of Health and Human Services, NIH hosted a public forum on implementation activities associated with the National Pain Strategy, a plan for improving pain management in the US (read the final report(pdf, 263 KB)).
NINDS kicked off 2017 by announcing 30 inaugural recipients of the R35 Research Program Award (RPA). Unlike R01 awards, which provide support for up to five years for a specific set of experiments, the RPA funds an investigator’s laboratory for up to eight years, enabling the pursuit of long-range, innovative research. The awards cover a spectrum of topics, but tend to focus more on fundamental and disease-related basic research, such as elucidating the structure of the enigmatic fusion pore, and defining neural development processes that can be leveraged to identify genetic risk factors for neurological disorders.
In the fall of 2017, with guidance from a Trans-NIH Working Group, NIH invested over $7 million to launch a consortium of centers to conduct coordinated scientific research on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS), a complex disease that has so far eluded understanding. The heterogeneous presentation of ME/CFS requires large-scale, trans-NIH efforts to provide an evidence base for effective therapy development. In addition to establishing the Consortium, the NIH awarded Administrative Supplements to expand ME/CFS research in current grants and initiated ME/CFS research at the NIH Clinical Center in Bethesda, Maryland, led by NINDS Clinical Director, Dr. Avi Nath.
Now in its fourth full year, the BRAIN Initiative® is paving a new course of fundamental neuroscience research. In 2017, the Initiative funded 110 new awards, bolstered by funds from the 21st Century Cures Act. These new awards support cross-disciplinary, team-based science and cutting-edge technology development to understand neural circuit function, as well the first set of awards for dedicated neuroethics research. As part of this investment, NIH made nine awards that together formed the BRAIN Initiative Cell Census Network, which will create a comprehensive 3D mouse brain cell atlas, generate reference brain cell atlases from postmortem healthy adult human and non-human primate samples, and build an integrative data center. To date, more than 275 publications have described new BRAIN Initiative-related advances and techniques for studying the brain in action. As the Initiative nears its halfway point, the NIH is planning to form an external working group to review progress to date and provide an updated scientific vision to guide the second half of the Initiative.
Congressional appropriations to the NIH in Fiscal Years 2016 and 2017 provided a generous boost in funding for Alzheimer's Disease (AD) and AD-related dementias (ADRD). As a lead NIH Institute for ADRD research, NINDS collaborates with the National Institute on Aging (NIA) to create new funding opportunity announcements (FOAs) and extend the payline for meritorious investigator-initiated projects on AD/ADRD. We urge the research community to join in our efforts to accelerate scientific progress toward reducing the enormous burden and cost of dementia. The National Advisory Neurological Disorders and Stroke Council (NANDSC), the Advisory Council to NINDS, approved several AD/ADRD research concepts to be further developed into new programs and initiatives, based on input from ADRD summits.
Separately, NINDS, Celgene, Verily, Pfizer, GlaxoSmithKline, Sanofi, the Michael J. Fox Foundation (MJFF), and the Foundation for NIH (FNIH) are launching the Accelerating Medicines Partnership for Parkinson’s Disease (AMP-PD). This new addition to the AMP program aims to identify and validate biomarkers and new therapeutic targets for PD, and it will leverage a treasure trove of data and resources supported over the past several years by NINDS and others.
As another example of collaboration, along with the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), NINDS also released the 2017 Strategic Plan for Cerebral Palsy Research, which outlines a set of research priorities to advance our understanding and treatment of cerebral palsy, working together with our partners as we seek to make meaningful progress in cerebral palsy research.
Finally, and as a reminder of the promise of research supported by NINDS and others, 2017 brought new FDA-approved therapies for neurological disorders, including an enzyme replacement therapy for a form of Batten disease, the first treatments for tardive dyskinesia and chorea associated with Huntington’s disease, and new drugs for amyotrophic lateral sclerosis (ALS) and multiple sclerosis (MS). A number of promising new therapies, including several supported through NINDS translational research programs, have also entered clinical trials. Overall, NINDS extramural and intramural investigators reported more important advances across basic, translational, and clinical neuroscience than I can describe here, but I invite you to look back on a small selection of highlights below.
These efforts could not advance without leaders at the helm. In 2017, NINDS welcomed three individuals to leadership roles: Dr. Nina Schor as Deputy Director, Dr. Amir Tamiz as Director of Translational Research, and Dr. Clinton Wright as Director of Clinical Research. Moving into 2018, we look forward to appointing a new Scientific Director and Director of Neuroscience to the NINDS team. With these new leaders in place, we look forward to an even more productive 2018, as we continue our mission of seeking fundamental knowledge about the nervous system and using that knowledge to reduce the burden of neurological disease.
Selected 2017 NINDS Science Advances
CLINICAL
- NINDS-funded research links midlife cardiovascular risk factors to dementia. With an aging population, dementia is a major health concern. A long-term study in over 15,000 participants found that middle aged Americans who have vascular risk factors, including diabetes, high blood pressure and smoking, have a greater chance of suffering from dementia later in life. The findings add to a growing body of evidence linking cardiovascular health to brain health and suggest that managing vascular risk factors in middle age may help prevent the development of dementia. See article. See NINDS Press Release. See more.
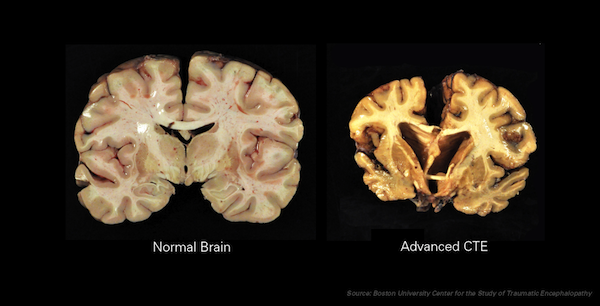
- Largest study to date documents chronic traumatic encephalopathy (CTE) in former American football players. CTE is a delayed neurodegenerative disorder that results from repetitive mild injury to the brain. Several individually small studies have described brain pathology consistent with CTE in postmortem brains from former athletes exposed to sports-related head trauma. This study provided alarming confirmation on a larger scale, with CTE found in 177 of 202 (87%) former American football players whose brains were donated for research, including 117 of 119 (98%) former professional players. See article.
- Silent seizures may contribute to cognitive decline in Alzheimer’s disease. An increased risk of seizures has been viewed as a late-stage phenomenon in Alzheimer’s disease (AD). In this study, researchers recorded brain activity in two patients with earlier-stage AD using electrodes inserted near the hippocampus, a region critical for memory that is affected in AD and commonly involved in epilepsy. The recordings revealed seizure activity without convulsions or other observable events, and these “silent” seizures occurred during sleep, when they could disrupt memory consolidation. While small, this study builds on prior research describing seizure-like activity in AD animal models and in patients, where it was associated with more rapid cognitive decline. Together, these studies raise intriguing questions about whether silent seizures contribute to AD progression and whether they may be a target for treatment. See article.
CLINICAL/TRANSLATIONAL
- Gene-editing therapies move from promise to reality. Antisense oligonucleotide (ASO) therapies employ sequences of nucleotides (the letters in the genetic code) designed to bind specific regions of a gene and modify its expression. This approach led to the FDA approval of an ASO therapy for spinal muscular atrophy (SMA) in late 2016. NINDS-funded investigator Don Cleveland, PhD, received a 2018 Breakthrough Prize, for his work to understand an inherited type of amyotrophic lateral sclerosis (ALS) and his role in developing ASO therapies now in clinical trials for ALS and Huntington’s disease. (See UCSD Press Release.) As additional examples, NINDS-funded researchers reported exciting results in animal models with an ASO for spinocerebellar ataxia (see article) that also affects disease mechanisms in ALS (see article and NINDS Press Release); and others are developing an ASO to reduce levels of tau proteins, which contribute to several neurodegenerative disorders (see article and NINDS Press Release).
BASIC/TRANSLATIONAL
- Noninvasive Deep Brain Stimulation via temporally interfering electric fields. Brain stimulation approaches are important in neuroscience research and for the treatment of disorders such as Parkinson’s disease, depression, and epilepsy. However, current methods for human brain stimulation present several limitations: invasive approaches use electrodes inserted into the brain, and non-invasive approaches fail to reach regions beyond the brain’s surface. Investigators supported by NINDS and others have described a new approach to non-invasive deep brain stimulation based on a concept known as beat frequency. When two oscillating electric fields coincide, they create a combined envelope that ‘beats’ at a frequency equal to the difference between the frequencies of the original fields. The investigators showed that they can can stimulate neurons in mice with this approach. Moreover, by manipulating the intensities and locations of applied fields, they could steer intersections with effective beat frequencies to different brain regions. The investigators hope further development will allow this approach to be tested in humans. Devising innovative new approaches to non-invasive, selective brain stimulation is also a goal of the NIH BRAIN initiative, and these results point to exciting progress on the horizon. See article.
BASIC/BASIC DISEASE
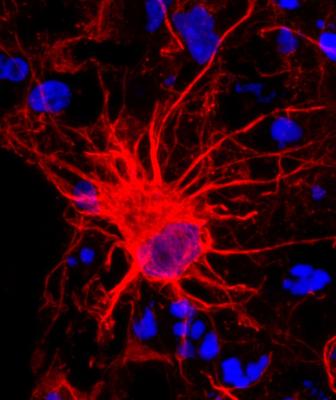
- Reactive astrocytes can be deadly. Astrocytes are a type of non-neuronal glia cell in the central nervous system with important roles supporting neural and immune function. Brain injury and disease can cause astrocytes to enter a 'reactive' state, and researchers have debated the extent to which reactive astrocytes are helpful or harmful. In this study, NINDS-funded investigators describe a novel subtype of reactive astrocytes that lose supportive and survival-promoting functions and instead induce cell death. These newly characterized astrocytes were abundant in postmortem human brain samples from individuals with Alzheimer’s disease, Huntington’s disease, ALS, Parkinson's disease, and multiple sclerosis, suggesting their presence may contribute to neurodegenerative disease processes and opening new avenues in the search for neuroprotective interventions. The study’s last author, Ben A. Barres, Ph.D., sadly passed away at the end of 2017. His research transformed our understanding of glia cells, and his dedication to mentorship, equality, and diversity strengthened the neuroscience community in immeasurable ways. See article.
- Novel mechanism in neuronal response to toxic stress. An NIH-funded research team has discovered a new process that helps neurons clear out toxic protein aggregates and organelles such as mitochondria that no longer function properly. Through studies in the round worm C. elegans, the researchers found that neurons extrude defective mitochondria and toxic clumps of protein by generating large vesicles they call exophers. Over time, exopher content appears to be degraded or engulfed by other cells. The researchers suggest that this process, when limited through aging or other factors, may contribute to neurodegenerative disease. See article.
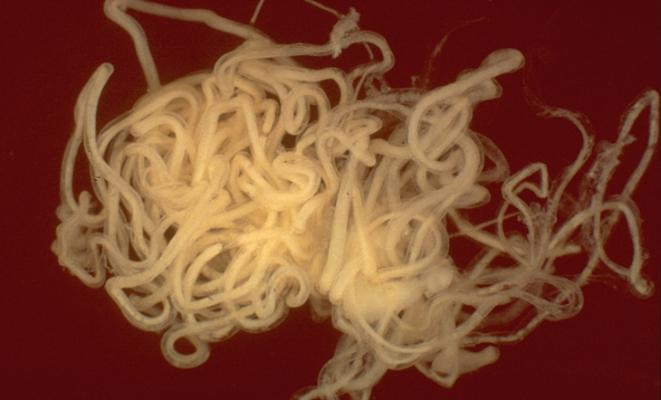
- Possible cause discovered for mysterious pediatric epilepsy. Nodding syndrome is a devastating form of pediatric epilepsy found in areas of east Africa and characterized by head nodding, seizures, cognitive deterioration, and stunted growth. Many studies have associated Nodding syndrome with the parasitic worm Onchocerca volvulus, but whether and how the worm caused the disorder had remained a mystery. In this study, NINDS intramural investigator and Clinical Director Avindra Nath, M.D., and his colleagues compared serum samples from patients with Nodding syndrome and healthy controls from the same village in Uganda. In those with Nodding syndrome, they found antibodies to a protein normally expressed in the brain called leiomodin-1. The antibodies were toxic to neurons grown in the lab, and they cross-reacted with antigens from the O. volvulus worm. The results point to an autoimmune mechanism for the disease, whereby antibodies generated by the immune system to attack a harmful intruder inadvertently attack the body’s own proteins as well. This research may inform better ways to diagnose, treat, and prevent Nodding syndrome, and potentially other epilepsies with autoimmune causes. See article. See NINDS Press Release.
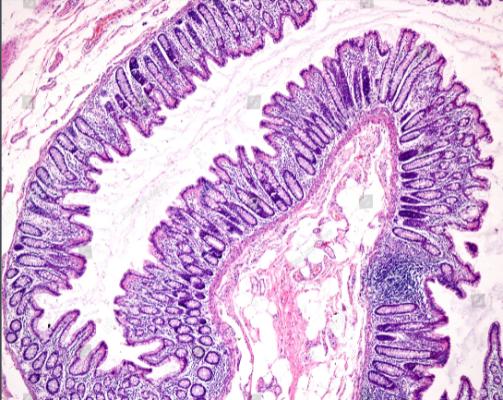
- Researchers identify gut chemosensors that couple to neural pathways. The lining of the gut is an important site for transmitting information about dietary, microbial, and inflammatory agents to the nervous system. Exactly how such signaling occurs has remained unclear, in part because cells suspected to play a major role (enterochromaffin (EC) cells) are sparse and hard for researchers to access. The authors of this study exploited a new technique to grow organoids in the lab, which allowed them to study EC cells directly. They found that EC cells express receptors that detect irritants, microbial metabolites, and stress-response hormones. EC activation by such agents triggers them to release the neurotransmitter serotonin, which in turn modulates sensory nerves through synaptic connections. The findings shed new light on how the gut conveys information to the nervous system, with implications for understanding bowel disorders and a wider range of processes influenced by the gut-brain axis. See article.
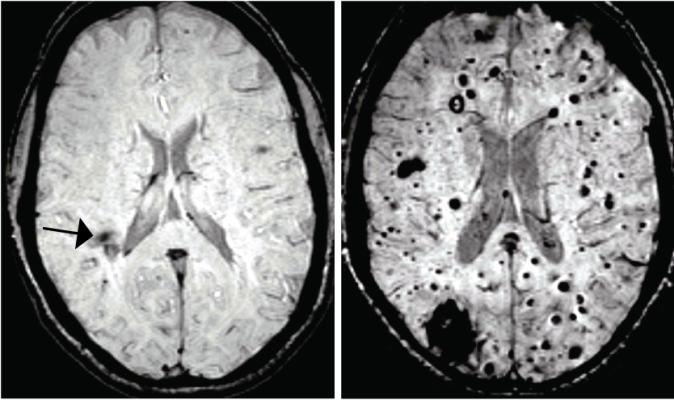
- Researchers connect brain blood vessel lesions to intestinal bacteria. Cerebral cavernous malformations (CCMs) are clusters of dilated, thin-walled blood vessels that can lead to seizures or stroke when blood leaks into surrounding brain tissue. Scientists studying the mechanisms that cause CCMs found an unexpected link to bacteria in the body. The researchers observed that mice with CCMs also had bacterial infections, and further study revealed that mice raised in a germ-free environment were protected from CCM development, as were mice treated with antibiotics to reset their internal bacteria. CCM formation was accelerated by inducing infection or activating a signaling pathway triggered by bacterial infection, and blocking this pathway prevented CCM formation. These findings may help explain the variability seen across people genetically predisposed to develop CCMs and could inform strategies for prevention. More broadly, they add to growing recognition of the many ways our microbiome – the community of bacteria residing in our bodies – contributes to health and disease. See article. See NINDS Press Release.