If you know anyone with dementia, you probably understand how devastating it is to those affected and their families. Alzheimer’s Disease (AD), the most common and widely-diagnosed neurodegenerative disorder, affects more than 5 million people in the United States. Other related forms of dementia also affect millions of Americans and commonly co-occur with typical AD, representing a significant and increasing burden on public health. Known as AD-related dementias (ADRD), these include Lewy body dementia (LBD), frontotemporal dementia (FTD), vascular cognitive impairment/dementia (VCI/D), and mixed dementias. Notably, cerebrovascular disease is exceedingly common in older populations who are diagnosed with AD and in persons with disabling age-related cognitive decline.
Congressional appropriations to the NIH in Fiscal Years (FY) 2016 and 2017 provided a generous boost in funding for AD and ADRD research – an additional $350 million in FY2016 and an additional $400 million in FY2017. As a lead NIH Institute for research on ADRD, NINDS collaborates with the National Institute on Aging (NIA), the lead NIH Institute for AD research, to put these funds to work by creating new funding opportunity announcements (FOAs) and extending the payline for meritorious investigator-initiated projects on AD/ADRD. Going forward, NINDS and NIA will continue to partner in AD/ADRD research planning and implementation, and we urge the research community to join in our efforts to accelerate scientific progress toward reducing the enormous burden and cost of dementia.
The need for scientific effort to reduce the burden of Alzheimer’s Disease and Related Dementias
Currently, there are no disease-modifying treatments available for AD, LBD, FTD, vascular, and mixed dementias, and the lack of optimal clinical diagnostic tools for dementia makes it even more challenging to find effective treatments or guide patient care. We have previously noted the importance of controlling hypertension, the primary driver of cerebrovascular disease, for stroke prevention and healthy brain aging. A recent report from the National Academies of Sciences, Engineering, and Medicine, “Preventing Cognitive Decline and Dementia: A Way Forward,” concluded that the “evidence suggests that managing blood pressure for people with hypertension, particularly during midlife (ages 35 to 65 years), is supported by encouraging but inconclusive evidence for preventing, delaying, and slowing clinical Alzheimer’s-type dementia.” While promising, we still have much to learn about the interaction between vascular and AD pathology, how to leverage cardio- and cerebro-vascular health measures to prevent dementia, and moreover, how to prevent or halt other types of dementia.
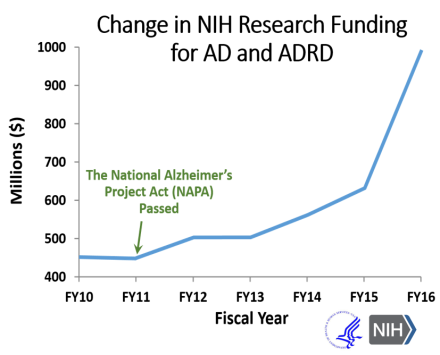
As the U.S. population ages, we need to reduce the tremendous health and economic burdens that face individuals with dementia, their loved ones, caregivers, and society. Recognizing this urgent need, Congress passed and President Obama signed the National Alzheimer’s Project Act (NAPA) into law in 2011, as a coordinated national effort to fight AD/ADRD. Since then, the NIH budget for AD/ADRD research has more than doubled, including the significant increase in funds in FY2016 and FY2017.
Extended Payline for AD/ADRD Investigator-initiated Research
Since FY2016, the NIA has utilized a higher pay line for AD/ADRD research to support investigator-initiated research grants, which it extends to NINDS and other Institutes and Centers for applications that meet this bar. This pay line is considerably higher than the pay line for other types of research in either NINDS or NIA. NINDS remains very interested in grant submissions focused on innovative ideas that could lead to prevention or effective treatment of AD/ADRD.
Future Opportunities
NINDS is also actively developing concepts for new initiatives to address research priorities identified through periodic NIH-hosted ADRD Summits (ADRD 2013(pdf, 980 KB) & ADRD 2016), where researchers, clinicians, patients, caregivers, and advocates gather to assess scientific progress and generate research recommendations. Some of these prioritized scientific opportunities include improving understanding of disease mechanisms in ADRD, determining the presence and significance of co-morbid brain pathologies in individuals with AD/ADRD, and improving clinical detection and diagnoses of AD/ADRD. Last year, the National Advisory Neurological Disorders and Stroke Council (NANDSC), the Advisory Council to NINDS, approved several AD/ADRD research concepts to be further developed into new programs and initiatives as funds become available. Six examples are shown below.
NINDS’s ADRD research concepts for FY2018
- Center without Walls (CWOW) for ADRD Radioligand Development and Testing – This would support the development of imaging ligands for Tau, TDP43, synuclein, and synaptic and neuroinflammatory markers that will enable better patient stratification, diagnosis, and tracking of disease progression in FTD , LBD and dementias with mixed etiologies. Current Tau PET ligands, which were identified by screening for binding in AD tissue, lack the specificity and sensitivity required for the tau-related ADRDs. The research focus could include screening of existing and derivative compounds against tau, as well as the other protein aggregates found in FTD, LBD, and Parkinson’s Disease human tissue. The Center could support the synthesis of ligands, analysis of ligands in human tissue, and first-in-human studies.
- Planning Grants for Clinical Trials to develop treatments for LBD – To address the lack of treatments for the disabling clinical features of LBD, this concept was developed to provide support to convene a clinical trial team that will develop the rationale and research design required for the submission of an NIH clinical trial grant application.
- Mechanisms of diffuse white matter disease and small vessel pathology in Vascular Contributions to Cognitive Impairment and Dementia (VCID) – This would support hypothesis-testing research to elucidate cellular and molecular mechanisms that underlie diffuse white matter disease and small vessel disease in VCID. The ultimate goal is to develop a rigorous mechanistic understanding that will enable future translational and clinical studies in VCI/D, AD, and other mixed etiology dementias that include diffuse white matter disease and small vessel pathology.
- LBD Center without Walls – A collaborative, multidisciplinary and possibly multi-national research group would be developed to elucidate the neuropathological mechanisms that result in the clinical pathology characteristic of LBD. The Centers will focus on understanding interactions between tau and alpha-synuclein and how these contribute to selective cell and circuit vulnerabilities.
- Pathway and target discovery for ADRD – This would support the discovery and validation of new pathways, targets, and potential biomarkers related to the human biology of ADRD, and support hypothesis-driven research using enhanced bioinformatics for large scale data analysis and disease modeling. Researchers would be encouraged to use existing cohorts, data, and well-characterized biological resources.
- Understanding the structural biology of ADRD-associated proteins – This would leverage recent advances in Cryo-electron microscopy (Cryo-EM) to identify structural forms of ADRD-associated proteins present in human brain tissue. Understanding structural differences in strains or isoforms of proteins like tau, alpha-synuclein, and TPD43 will support and advance the basic biology underlying ADRD, as well as contribute to the development of better diagnostic tools and therapies for these diseases.
Now is truly an exciting time for dementia research, and our program staff is working hard to transform these concepts into real funding announcements. In the meantime, I strongly encourage the research community to develop ideas and be prepared to take advantage of upcoming, unprecedented opportunities to advance ADRD research.
