
Neurons are nerve cells that send messages all over your body to allow you to do everything from breathing to talking, eating, walking, and thinking. Until recently, most neuroscientists (scientists who study the brain) thought we were born with all the neurons we were ever going to have. As children, we might grow some new neurons to help build the pathways—called neural circuits—that act as information highways between different areas of the brain. However, scientists believed that once a neural circuit was in place, adding any new neurons would change the flow of information and break the brain’s communication system
In 1962, scientist Joseph Altman challenged this belief when he saw evidence of neurogenesis (the birth of neurons) in a region of the adult rat brain called the hippocampus. He later reported that newborn neurons traveled from their birthplace in the hippocampus to other parts of the brain. In 1979, another scientist, Michael Kaplan, confirmed Altman’s findings in the rat brain; and in 1983, he found special kinds of cells—called neural precursor cells—with the ability to become brain cells like neurons, in adult monkeys.
These discoveries about neurogenesis in the adult brain were surprising to other researchers who thought they were not true in humans. Fortunately, in the early 1980s, a scientist trying to understand how birds learn to sing began to see how neurogenesis in the adult brain might make sense. In a series of experiments, Fernando Nottebohm and his research team showed that the numbers of neurons in the forebrains (areas controlling complex behaviors) of male canaries dramatically increased during the mating season, when the birds learn new songs to attract females.

Why did these bird brains add neurons at such an important time in learning? Nottebohm believed it was because newborn neurons helped store new song patterns within the pathways of the forebrain. These new neurons made learning new songs possible! If birds made new neurons to help them remember and learn, Nottebohm thought the brains of mammals—like humans—might too.
Other scientists, like Elizabeth Gould, later found evidence of newborn neurons in a distinct area of the brain in monkeys. And Fred Gage and Peter Eriksson showed that the adult human brain produced new neurons in a similar area.
Many aspects of neurogenesis in the adult human brain are still not well understood, including how it impacts the brain and its functions. Still, scientists are intrigued by current research on neurogenesis and the possible role of new neurons in the adult brain for learning and memory.
The Architecture of the Neuron
The central nervous system (which includes the brain and spinal cord) is made up of two basic types of cells:
- Neurons, the nerve cells that send and receive signals
- Glia, cells that provide structure in the brain
In some parts of the brain, there are many more glia than neurons, but neurons are the key players in the brain.
Neurons are information messengers. They use electrical and chemical signals to send information between different areas of the brain, as well as between the brain, the spinal cord, and the entire body. Everything we think, feel, and do would be impossible without the work of neurons and their support cells, the glial cells called astrocytes and oligodendrocytes.
A neuron has three basic parts: a cell body, an axon, and dendrites. Within the cell body is a nucleus, which controls the cell’s activities and contains the cell’s genetic material. The axon looks like a long tail and sends messages from the cell. Dendrites look like branches of a tree and receive messages for the cell. Neurons communicate with each other by sending chemicals, called neurotransmitters, across a tiny space called a synapse, between the axons and dendrites of nearby neurons.

There are three kinds of neurons:
- Sensory neurons carry information from the sense organs (such as the eyes and ears) to the brain.
- Motor neurons control voluntary muscle activity, such as walking and talking, and carry messages from nerve cells in the brain to the muscles.
- Other neurons, called interneurons, make connections between sensory and motor neurons.

Scientists think that neurons are the most diverse kinds of cells in the body. Within these three kinds of neurons are hundreds of different types, each able to send and receive messages in different ways.
How these neurons communicate with each other by making connections is what makes each of us unique in how we think, feel, and act.
Neurogenesis
Many neuroscientists disagree about how many and how often new neurons are created in the brain. Most of the brain’s neurons are already created by the time we’re born, but there is evidence to support the theory that neurogenesis is a lifelong process.
Neurons are born in areas of the brain that are full of neural stem cells, or precursor cells. Stem cells have the potential to make most, if not all, of the different types of neurons and glia found in the brain.
Neuroscientists have observed how neural stem cells behave during experiments in the laboratory. Although this may not be exactly how these cells act when they’re in the brain, it gives us information about how they might function when they’re in the brains of humans or other animals.
The science of stem cells is still very new and could change with additional discoveries, but researchers have learned enough to be able to describe how neural stem cells create the other cells of the brain.
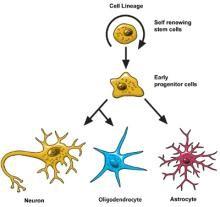
Neural stem cells increase by dividing in two. Then they can become either two new stem cells, or two early progenitor cells (parent cells to new neurons or glia), or one of each.
When a stem cell divides to produce another stem cell, it is said to self-renew. This new cell has the potential to make more stem cells.
When a stem cell divides to produce an early progenitor cell, it is said to differentiate. Differentiation means that the new cell is more specialized in how it’s formed and what it can do.
Early progenitor cells can make other progenitor cells, self-renew like stem cells, or can change in either of two ways. One way will make new astrocytes. The other way will make neurons or oligodendrocytes.
Neuronal Migration
Once a neuron is born, it must travel to the place in the brain where it will do its work.
Scientists have seen that neurons use at least two different methods to travel:
- Some neurons travel, or migrate, by following the long fibers of cells called radial glia. These fibers stretch from the inner layers to the outer layers of the brain. Neurons glide along the fibers until they reach their destination.
- Neurons also travel by using chemical signals. Scientists have found special molecules on the surface of neurons—adhesion molecules—that attach to similar molecules on nearby glial cells or nerve axons. These chemical signals guide the neurons to their final location.
Not all neurons are successful in their journey. Scientists think that only a third reach their destination. Some cells die in development or while traveling.
Other neurons survive the trip but end up in the wrong place. Accidental changes (called mutations) in the genes that control migration create brain areas of misplaced or oddly formed neurons that can cause disorders like childhood epilepsy. Some researchers think that certain disorders, such as schizophrenia and dyslexia, are partly the result of misguided neurons.
Differentiation
Once a neuron reaches its destination, it must settle into work. There is still a lot that scientists don’t understand about this part of neurogenesis, called differentiation.
Neurons are responsible for sending and receiving neurotransmitters—chemicals that carry information between brain cells.
Depending on its location, a neuron can perform the job of a sensory neuron, a motor neuron, or an interneuron. Each of these types of neurons sends and receives specific neurotransmitters.
In the developing brain, a neuron depends on signals from other cells to determine its shape and location, the kind of transmitter it produces, and the other neurons it will connect to. These freshly born cells create neural circuits—or information pathways connecting neurons to other neurons—that will be in place throughout adulthood.
But in the adult brain, neural circuits are already developed, and neurons must find a way to fit in. As new neurons settle in, they start to look like the surrounding cells. They develop axons and dendrites and begin to communicate with their neighbors through synapses.
Death of a Neuron
Although neurons are the longest living cells in the body, large numbers of them die during migration and differentiation. The lives of some neurons can take strange turns. Some diseases of the brain are the result of the unnatural deaths of neurons.
- In Parkinson’s disease, neurons that produce the neurotransmitter dopamine die off in the basal ganglia, an area of the brain that controls body movements. This causes people with this disease to experience tremor, to move more slowly, and to have problems with balance.
- In Huntington’s disease, a genetic mutation causes neurons to create too much of a neurotransmitter called glutamate, which kills neurons in the basal ganglia. As a result, people twist and move uncontrollably, and over time, they lose the ability to do many everyday tasks like walking and eating.
- In Alzheimer’s disease, unusual proteins build up in and around neurons in the neocortex and hippocampus, the parts of the brain that control memory. When these neurons die, people lose their abilities to remember and do everyday tasks.
- Physical damage to the brain and the spinal cord can also kill or disable neurons. Damage to the brain caused by shaking or hitting the head, or because of a stroke, can kill neurons immediately or slowly, starving them of the oxygen and nutrients they need to survive.
- A spinal cord injury can cut off communication between the brain and the muscles. When neurons lose their connection to the axons located below the site of injury, the neurons may still live, but they lose their ability to communicate.
The Future: What's Next in Neuron Research?
Scientists hope that by understanding more about the life and death of neurons, they can develop new treatments, and possibly even cures, for brain diseases and disorders that affect the lives of millions.
The most current research suggests that neural stem cells can generate many, if not all, of the different types of neurons found in the brain and the nervous system. Learning how to manipulate these stem cells in the laboratory into specific types of neurons could produce a fresh supply of brain cells to replace those that have died or been damaged.
Therapies could also be created that may make it possible to repair, reshape, and renew the brain from within.
