The Clinical Trial Journey
When you participate in a clinical trial, you can learn about your own health and help researchers discover new ways to prevent or treat health conditions—now, and for generations to come.





































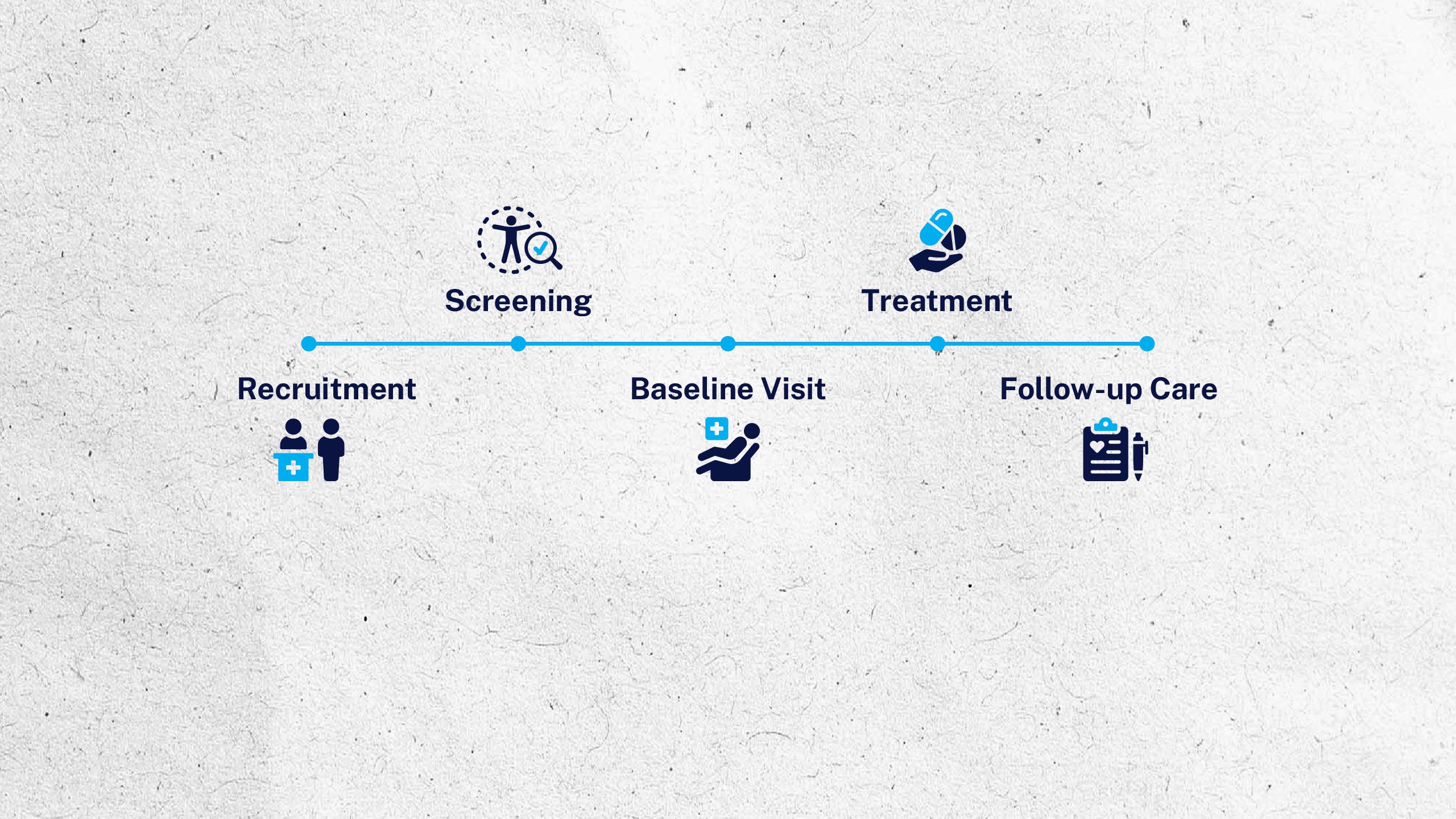
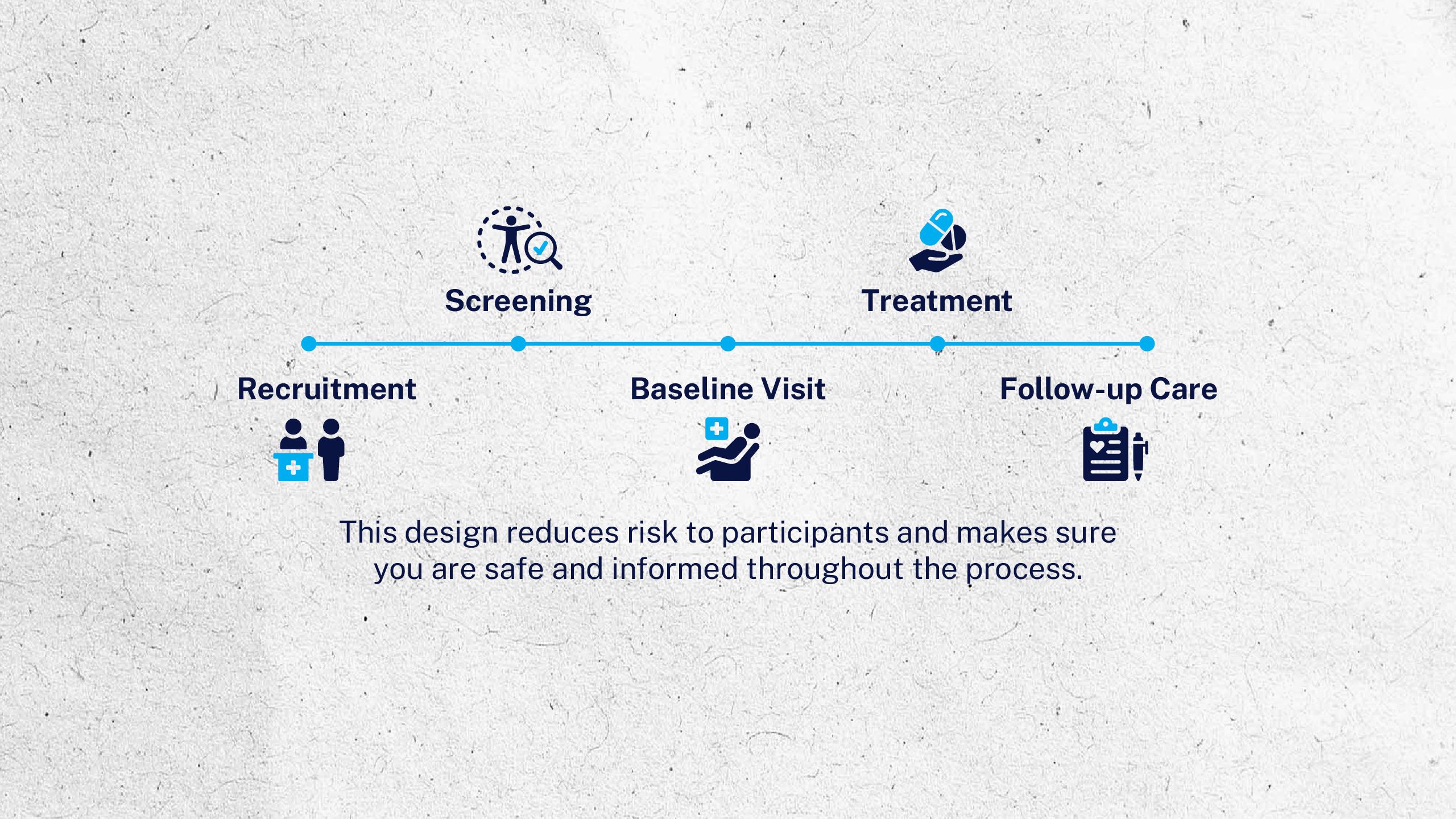




To learn more about clinical research and how to participate in a trial or study, visit ninds.nih.gov/clinical-trials.




