General Information
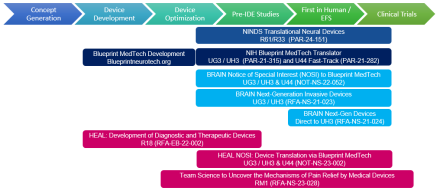
The Translational Devices program provides support for the development, optimization, translation, and first-in-human testing of therapeutic and diagnostic devices for disorders that affect the nervous or neuromuscular systems. The program consists of funding opportunities for investigator-initiated projects at NINDS (PAR-24-151) and through related trans-NIH programs.
Closed Funding Announcements
- RFA-NS-21-021 (UG3/UH3) - View funded projects under this NOFO
- RFA-NS-21-022 (U44) - View funded projects under this NOFO
- RFA-NS-18-011 (UG3/UH3) - View funded projects under this NOFO
- RFA-NS-18-012 (U44) - View funded projects under this NOFO
Companion Trans-NIH Device Programs
Blueprint MedTech
The overarching goal of the Blueprint MedTech program is to accelerate patient access to groundbreaking, safe, and effective medical devices. The program supports the translation of groundbreaking neurotechnologies from early stages through first-in-human studies by providing non-dilutive funding and additional resources to de-risk and develop therapeutic, and diagnostic devices for disorders that affect the nervous or neuromuscular systems.
For more information visit the Blueprint MedTech website.
Open Funding Announcements
- Neurotechnology Development through the MedTech Incubator Hubs
- PAR-21-315 (UG3/UH3) - Blueprint MedTech Translator
- PAR-21-282 (U44) - Blueprint MedTech Small Business Translator
Closed Funding Announcements
Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative
The Next-Generation Invasive Devices for Recording and Modulation in the Human Central Nervous System program encourages applicants to pursue milestone-driven translational and clinical projects for implantable recording and/or stimulating devices to treat central nervous system (CNS) disorders and better understand the human brain.
For more information visit the BRAIN Initiative website.
Open Funding Announcements
- NOT-NS-22-052 - Notice of Special Interest in the Blueprint MedTech program
- RFA-NS-21-023 (UG3/UH3) - Translation of Next-Generation Invasive Devices
- RFA-NS-21-024 (UH3) - Clinical Studies of Next-Generation Invasive Devices
Closed Funding Announcements
- RFA-NS-18-021 (UG3/UH3) - View funded projects under this NOFO
- RFA-NS-18-022 (U44)
- RFA-NS-18-023 (UH3) - View funded projects under this NOFO
Helping to End Addiction Long-Term (HEAL)
The Translating Discoveries into Effective Devices to Treat Pain program fosters the development of next-generation medical devices to diagnose and treat pain by supporting preclinical development of safe, effective, and non-addictive devices and approaches – providing treatment options for those who have no other effective ways to manage their pain. The program also supports the translation of promising devices into early-stage clinical trials that inform the functionality, final design, safety, and/or efficacy of technologies. In addition, the program supports interdisciplinary team science to reveal mechanisms underlying device-based pain relief, identify physiological markers of pain, and determine how existing device-based therapeutic approaches affect neural targets.
For more information visit the HEAL Initiative website.
Open Funding Announcements
- RFA-EB-22-002 (R18) - Development of Diagnostic and Therapeutic Devices
- NOT-NS-23-002 (UG3/UH3 and U44) - Notice of Special Interest in the Blueprint MedTech program
- RFA-NS-23-028 (RM1) - Team Science Research on Device Mechanisms
Closed Funding Announcements
- RFA-EB-18-003 (U18) - View funded projects under this NOFO
- RFA-NS-19-016 (UG3/UH3) - View funded projects under this NOFO
- RFA-NS-19-017 (U44) - View funded projects under this NOFO
- RFA-NS-19-018 (UH3) - View funded projects under this NOFO
- RFA-NS-22-016 (RM1) - View funded projects under this NOFO
Stimulating Peripheral Activity to Relieve Conditions (SPARC)
The SPARC program seeks to accelerate development of therapeutic devices that modulate electrical activity in nerves to improve organ function. SPARC is generating maps and tools to identify and influence therapeutic targets that exist within the neural circuitry of a wide range of organs and tissues. This therapeutic strategy, also known as “bioelectronic medicine,” could offer new treatment options for diverse diseases and conditions such as hypertension, heart failure, gastrointestinal disorders, type II diabetes, inflammatory disorders, and more. The SPARC Human Open-Research Neural Engineering Technologies (HORNET) initiative is building a new ecosystem of open-source neuromodulation systems for exploratory and clinical neuromodulation studies in the peripheral nervous system, and for potential applicability in the central nervous system.
For more information visit the SPARC Program website.
Closed Funding Announcements
Education Program on Translational Devices
The purpose of this program is to develop and implement a short course focused on (1) steps required for successful neural medical device development, translation, and commercialization, (2) common technical and strategic challenges, and (3) best-practices and resources for each stage in the process.
Closed Funding Announcements
- NIH Blueprint for Neuroscience Research: RFA-NS-20-003 (R25) - View funded projects under this NOFO
- Trans-NIH Reissue: PAR-22-146 (R25) - Applications under review
- Participating Institutes: NINDS, NIBIB, NIDDK, NIDA, NCI
Program Snap Shots
NINDS Translational Neural Devices
- Clinical Study Optional
- Invasive or noninvasive
- CNS or PNS
- Diagnostic or Therapeutic
- Within NINDS Mission
- Budgets should rarely exceed:
- UG3: $1M DC/yr
- UH3: $1.5M DC/yr
BRAIN Initiative Next-Generation Devices
- Clinical Study Optional
- Invasive only
- CNS only
- Diagnostic or Therapeutic
- Within mission of a BRAIN IC
- Budgets should rarely exceed:
- UG3: $1M DC/yr
- UH3: $1.5M DC/yr
- All funding opportunities for investigator-initiated projects use cooperative agreement mechanisms and are milestone-driven. This type of mechanism involves NIH program staff's participation in developing the project milestones and plans, monitoring the research progress, and making go/no-go decisions.
- For projects that have completed all non-clinical testing necessary to obtain an IDE or obtained NSR designation from a local IRB, a straight-to-clinical option (UH3) are available in the BRAIN Initiative.
- Phased cooperative agreement mechanisms (UG3/UH3 and SBIR U44 Fast-Track) support pre-clinical/translational device activities, including translational bench and animal studies, and are expected to lead to an Investigation Device Exemption (IDE) from the U.S. Food and Drug Administration (FDA) to support a small clinical trial, or a Non-Significant Risk (NSR) designation from an Institutional Review Board (IRB) in the first phase. The subsequent phase supports the small clinical study or trial to collect safety and effectiveness data required to support a marketing application, or to inform final device design.
- For UH3 awards, IRB/FDA approval is required prior to issuance of the NOA. For UG3/UH3 and U44 awards, IRB/FDA approval is required prior to release of funds for the clinical studies.
All Applicants are strongly encouraged to contact translational devices staff at least two months prior to the receipt date.
Resources and Tools
Contacts
Nick Langhals, Ph.D. | Program Director, Team Lead
nick.langhals@nih.gov
Brooks Gross, Ph.D. | Program Director
brooks.gross@nih.gov
Eric Hudak, Ph.D. | Program Director
eric.hudak@nih.gov
Guangying Wu, Ph.D. | Program Director
guangying.wu@nih.gov
Megan Frankowski, Ph.D. | Program Officer
megan.frankowski@nih.gov
Elyse Kemmerer White, Ph.D. | Scientific Program Manager
elyse.white@nih.gov
Eric W. Atkinson, Ph.D. | Scientific Program Analyst
eric.atkinson@nih.gov
Pantea Moghimi, Ph.D. | Scientific Program Analyst
pantea.moghimi@nih.gov
Sarah Robinson Schwartz, Ph.D. | Health Program Specialist
sarah.robinsonschwartz@nih.gov
Nasim Vahidi, Ph.D. | Scientific Program Analyst
nasim.vahidi@nih.gov
Ashley Givens | Program Specialist
Ashley.givens2@nih.gov
Funding Opportunities
View All Translational Research Opportunities
Related Topics
