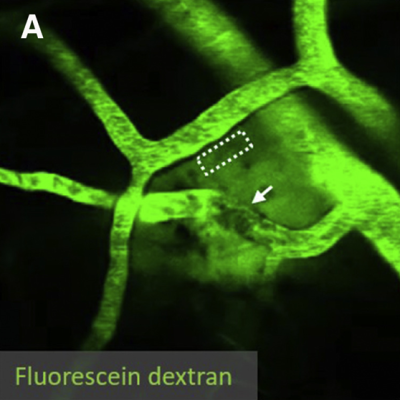
In Alzheimer’s disease and cerebral amyloid angiopathy (CAA), amyloid-beta protein fragments or plaques accumulate in the tissue and blood vessels of the brain, likely due to a faulty clearance mechanism. In mouse experiments, NINDS-funded researchers recently found that slow, spontaneous vessel pulsations, known as ‘vasomotion’, drive the clearance of waste products from the brain. In the study, scientists injected a fluorescently labeled substance called dextran into the brains of mice and used two-photon microscopy to image its clearance. They found that vasomotion was critical for clearing dextran and that increasing the amplitude of vessel pulsations could increase clearance. Vessel pulsations and clearance rates were also hindered in mice with CAA. These results highlight the importance of vasculature in the pathophysiology of Alzheimer’s disease and may inform new therapeutic strategies that delay or prevent the onset of Alzheimer’s and related diseases in humans.
Image: The paravascular clearance of fluorescently labeled dextran in the brains of awake mice was measured with two-photon microscopy.
Article: van Veluw SJ, et al., Vasomotion as a Driving Force for Paravascular Clearance in the Awake Mouse Brain. Neuron. February 5, 2020.
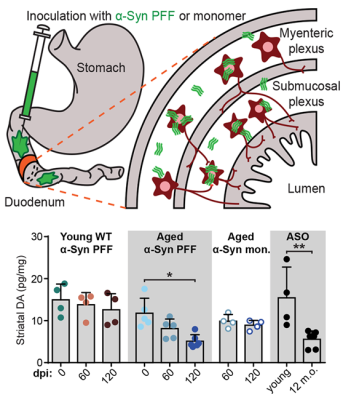
Parkinson’s disease (PD) is characterized by aggregates of the protein alpha-synuclein (α-Syn) in the brain. Growing evidence suggests that α-Syn begins accumulating in the gut and reaches the brain via autonomic nerve fibers. In a mouse model of PD, NINDS- and BRAIN Initiative-funded researchers demonstrated this gut-to-brain connection and found that α-Syn build up and its disease-related effects depend on age. In the study, Dr. Viviana Gradinaru and her team injected α-Syn into the gastrointestinal lining of young and aged mice and monitored the protein’s progression up to the brainstem and midbrain. In aged, but not young, mice they found gastrointestinal disturbances, motor symptoms, and other neurobiological deficits. Researchers also used a harmless virus engineered to deliver genes that encode the enzyme glucocerebrosidase (GCase), which breaks down α-Syn, to reduce aggregates and partially restore normal gut function. This work greatly advances our knowledge of the α-Syn pathology and may help inform gene therapies for PD in humans.
Image: Diagram of an injection of α-Syn preformed fibrils (PFF), an established model of the idiopathic disease process, or a monomer into the duodenum of the small intestine (top). Midbrain dopamine levels were depleted in aged mice that received gastrointestinal α-Syn PFF injections.
Article: Challis C et al., Gut-seeded α-synuclein fibrils promote gut dysfunction and brain pathology specifically in aged mice. Nature Neuroscience. March 23, 2020.
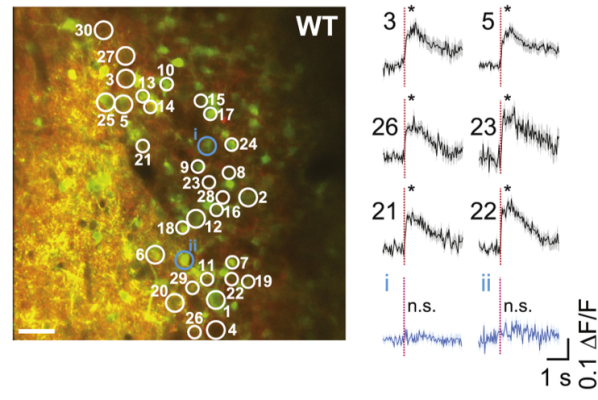
Sensory systems transform sensations from the external world into time-varying neural spiking activity. But until now, it was technologically difficult to determine which features of neuronal spiking guide behavior. In the mouse, smelling different odors leads to changes in the group of neurons activated, as well as when neurons are activated relative to each other (synchrony) and the start of odor inhalation (latency). To examine the relevance of each feature (i.e., rate, synchrony, and latency) to olfaction, NIH BRAIN Initiative-funded investigator Dr. Shy Shoham and his team developed a holographic two-photon optogenetic stimulation method with cellular and single-action-potential resolution. They applied their new technique to olfactory perception and found that mice can detect single spikes simultaneously in an incredibly precise manner and that synchrony, rather than latency, is key to sensory perception. This ultra-precise imaging approach has great potential in advancing our understanding of the neuronal dynamics of other sensory systems and behavior.
Image: Thirty olfactory bulb neurons were targeted for simultaneous two-photon photostimulation (white circles) and imaging (left). Average fluorescence responses, a proxy for neural activity, to photostimulation of the targeted (black) and non-targeted (blue) cells (right).
Article: Gill JV, et al. Precise Holographic Manipulation of Olfactory Circuits Reveals Coding Features Determining Perceptual Detection. Neuron. October 28, 2020.
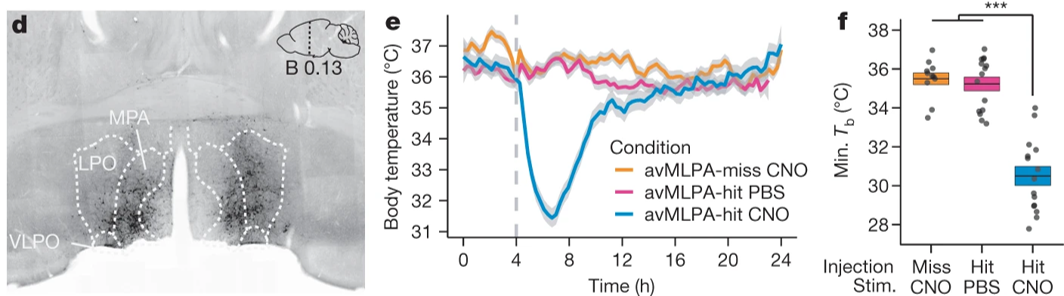
Torpor, or a state of decreased mental or physical activity, is an involuntary state that is essential for mammalian survival. But how homeothermic mammals initiate and regulate energy-conserving states remains largely unknown. Earlier this year, NINDS- and NIH BRAIN Initiative-funded researchers found that torpor is controlled by neurons in the medial and lateral preoptic area of the hypothalamus. Dr. Michael Greenberg and his team used excitatory Designer Receptor Exclusively Activated by Designer Drug (Gq-DREADDS) and the FosTRAP neuron ‘tagging’ or labeling approach during various torpor states in mice to discover these novel torpor-regulating neuronal subpopulations. This work will shine light on how torpor-regulating neural circuits control other adaptive biological processes such as thermoregulation and may inform related state changes in humans.
Image: Representative coronal section from an avMLPA-injected mouse (left). Chemogenetic activation of the anterior and ventral portions of the medial and lateral preoptic area (avMLPA) of the hypothalamus (blue) decreased core body temperature, inducing a state of torpor in mice. The same mice in a control condition (yellow) and mice lacking avMLPA TRAPed neurons (pink) did not show changes in torpor (right).
Article: Hrvatin S, et al., Neurons that regulate mouse torpor. Nature. June 11, 2020.
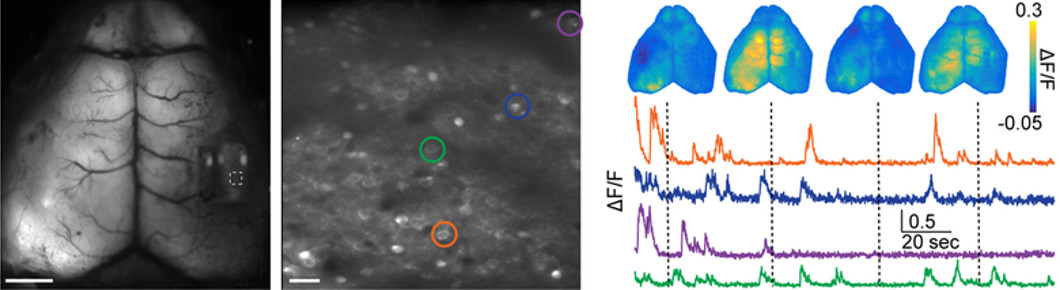
In the cortex, single neurons integrate signals from local circuits and long-range projections originating in distant brain regions. But linking local circuit function to global brain activity is technologically challenging. To address this, researchers funded by NINDS and the NIH BRAIN Initiative developed a method to simultaneously measure microcircuit activity of single neurons and the meso-scale activity in the mouse cortex. To achieve this, Dr. Michael Crair and his collaborators at Yale University unified two technologies: two-photon microscopy and mesoscopic imaging. Researchers leveraged their new tool to examine local and large-scale connections in the somatosensory cortex. They also imaged a notoriously sparse population of interneurons and identified a new ‘arousal network’ by linking single cell and network activity to whisking behavior. This powerful, multi-scale imaging tool will accelerate our ability to measure and understand brain architecture and function across scales.
Image: Fluorescence images from simultaneously acquired mesoscopic (left) and two-photon (middle) imaging in the mouse cortex. Example of averaged mesoscopic and cellular fluorescence levels, a proxy for neural activity, from the cells circled in the middle image.
Article: Barson D et al., Nat Methods. Simultaneous mesoscopic and two-photon imaging of neuronal activity in cortical circuits. November 4, 2019.
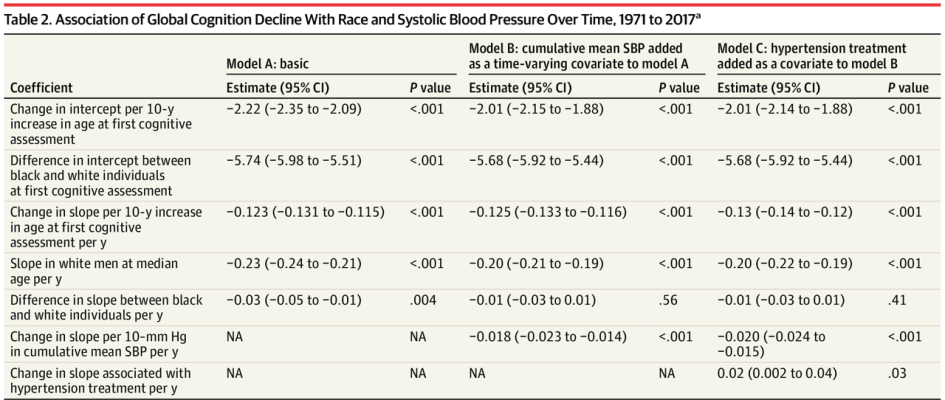
To determine whether cumulative blood pressure (BP) levels can explain racial differences in cognitive decline, researchers pooled individual participant data from five cohorts, including NOMAS, ARIC, CARDIA, Framingham, and the Cardiovascular Health Study, following them from January 1971 to December 2017. The primary outcome was change in global cognition, and secondary outcomes were change in memory and executive function. Black individuals’ higher BP levels were associated faster decline, compared with white individuals, in global cognition and memory but not executive function. This important longitudinal study demonstrates health disparities that are linked to racial differences in later-life cognitive decline.
Table: In following 19,378 individuals from five prospective cohort studies, researchers found an association of global cognition decline with race and systolic blood pressure across three models.
Article: Levine, D.A. et al. Association Between Blood Pressure and Later-Life Cognition Among Black and White Individuals. July 1, 2020, JAMA Neurology
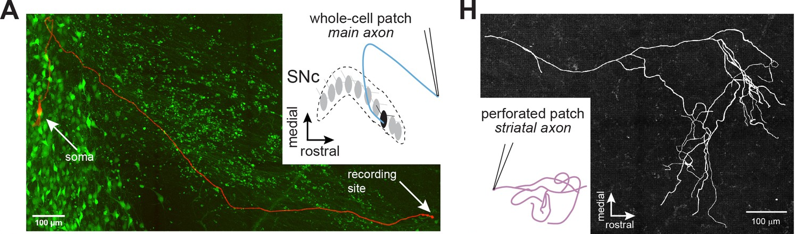
Axons of dopaminergic neurons innervate the striatum where they contribute to movement and reinforcement learning, but it remains unknown how GABA-A receptors modulate dopamine. In this technically innovative paper, researchers used whole-cell and perforated-patch recordings to test for GABA-A receptors on main dopaminergic neuron axons and branching processes within the striatum. The team found that GABA-A receptors are present on the axons of midbrain dopaminergic neurons, and that the receptors modulated propagation of action potentials in the axon. They also demonstrated that diazepam (Valium), a commonly prescribed broad-spectrum benzodiazepine, enhances axonal GABA-A receptors, resulting in shunting and subsequent inhibition of dopamine release. Together, these experiments reveal the mechanisms of GABA-A receptor modulation of dopamine release and provide new insight into the role of axonal GABA-A receptors in the actions of benzodiazepines in the striatum.
Image: Whole-cell and perforated-patch recordings were collected from dopamine neuron axons. (left) In whole-cell mode, axons were recorded with a connected soma. (right) Patched striatal axons were later reconstructed.
Article: Kraemer PF, et al. Axonal mechanisms mediating γ-aminobutyric acid receptor type A (GABA-A) inhibition of striatal dopamine release. Sept 1 2020, Elife.
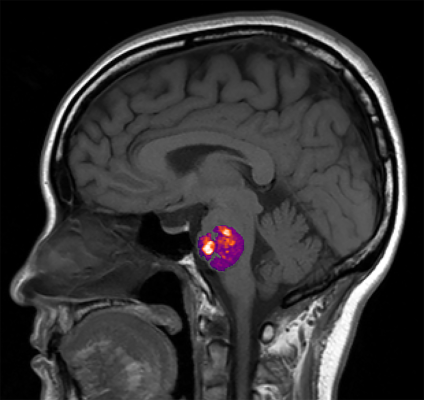
A growing body of evidence supports the role of gut bacteria in brain health. In a nationwide study, NIH-funded researchers led by a team at the University of Chicago examined the link between abnormal bundles of brittle blood vessels in the brain or spinal cord, known as cavernous angiomas (CA), and the composition of a person’s gut bacteria. Using advanced genomic analysis techniques, the researchers found that the relative abundance of three gut bacterial species distinguished CA patients from controls regardless of other factors. CA patients also showed more gram-negative bacteria, whereas controls had more gram-positive bacteria. These results provide the first demonstration in humans of a “permissive microbiome” associated with the formation of neurovascular lesions in the brain.
Image: An NIH funded study found a link between the appearance of abnormal, stroke-inducing blood vessel bundles, called cavernous angiomas, and the composition of a person’s gut bacteria. Courtesy of Awad lab University of Chicago, IL.
Article: Polster, S.P.; Sharma, A. et al. Permissive microbiome characterizes human subjects with a neurovascular disease cavernous angioma, May 27, 2020, Nature Communications
NIH Press Release:
Study ties stroke-related brain blood vessel abnormality to gut bacteria
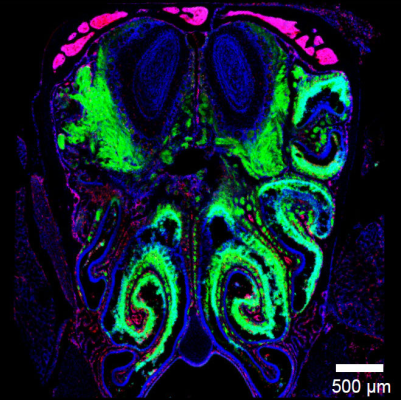
The location of nasal neurons and their exposure to the outside environment make them an easy target for infection by airborne viruses, but viral respiratory infections rarely make their way from the olfactory bulb to the rest of the brain, where they could cause potentially fatal encephalitis. Using fluorescent microscopy, the researchers led by Dorian McGavern, Ph.D., senior investigator at NINDS, found that a viral infection that started in the nose was halted right before it could spread from the olfactory bulb to the rest of the central nervous system. Dr. McGavern’s team showed that CD8 T cells, which are part of the immune system responsible for controlling viruses, can act as a front-line defense mechanism in protecting the brain after infection of nasal tissue. Additional experiments showed that microglia, immune cells within the central nervous system, took on an underappreciated role of helping the immune system recognize the virus and did so in a way that limited the damage to neurons themselves.
Image: Viral infection thwarted just outside the CNS. When virus (labeled in green) enters the nasal passages, its spread is abruptly halted just before entering the CNS (blue oval structures at the top of the image). Image courtesy of McGavern lab
Youtube video: Virus-specific CD8 T cells migrating across infected neurons that project to the olfactory bulb
Article: Moseman, EA et al. T cell engagement of cross-presenting microglia protects the brain from nasal virus infection. Science Immunology. June 5, 2020.
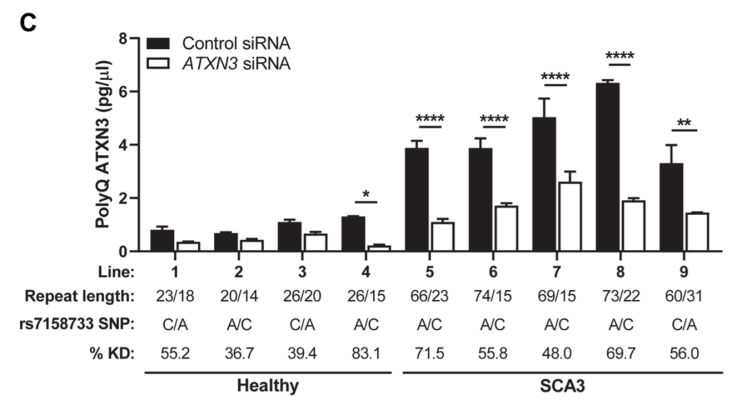
Spinocerebellar ataxia type 3 (SCA3) – caused by a repeat expanstion in the ataxin-3 gene (ATXN3) – is a devastating neurodegenerative disorder that results gait and limb ataxia as well as other movement dysfunction. Characterized by aggregation of polyglutamine (polyQ) ATXN3 protein aggregates, treatments have been difficult because of the lack of pharmacodynamic markers. Here, researchers developed an immunoassay that readily detects polyQ ATXN3 proteins in human biological fluids and discriminates SCA3 patients from healthy controls and individuals with other ataxias, showing that polyQ ATXN3 serves as a marker of target engagement in human fibroblasts. This scientific advance paves the way for identifying biomarkers in persons with spinocerebellar ataxia that could enable testing of therapeutics in phase clinical trials.
Image: To determine whether fibroblast lines could assess patient responses to ATXN3-targeting therapies, researchers grew four control and five SCA3 lines in parallel, then treated them with ATXN3 or control small interference RNAs (siRNAs). This treatment reduced polyQ ATXN3 levels in all five SCA3 lines.
Article: Prudencio M et al. Toward allele-specific targeting therapy and pharmacodynamic marker for spinocerebellar ataxia type 3. October 21, 2020, Science Translational Medicine.
Like many of you, I am looking forward to 2021 as a new year of possibility. As a country, we have faced challenges in 2020 that have been unlike anything we have ever experienced in decades. My heart goes out to all those who have experienced loss associated with the COVID-19 pandemic. We continue to learn more about the effects of SARS-CoV-2 and COVID-19 on the nervous system every day. Yet, when I look back on the year, I am struck – perhaps more so than anything else – by the dedication of our tenacious and resilient research community to meet those challenges and continue to create research progress.
The early days of the COVID-19 pandemic forced us at NIH to make quick decisions to support our applicants and grantees. Many Institutes and Centers, including NINDS, issued supplement opportunities to support researchers studying the SARS-CoV-2 virus in relation to their existing awards. We also issued administrative flexibilities and extensions of eligibility to accommodate individuals who are juggling additional demands during the pandemic. One of these supplement opportunities resulted in the establishment of a COVID-19 NeuroDatabank and NeuroBioBank at NYU Langone Health. As this database and biospecimen bank comes online, it will serve as a national resource to document and study the neurological complications of COVID-19. Interested researchers will be able to submit requests for the use of data and biosamples to NYU and we hope to understand better the neurologic complications and changes in neurological status caused by COVID-19. Further, we have been working with our colleagues across NIH to study post-acute COVID syndrome. We plan to incorporate research questions on the long-term effects of COVID into ongoing studies and are working on plans to establish additional prospective research plans. I personally learned a lot from the NIAID/NIH Workshop on Post-Acute Sequelae of COVID-19 (videocast day 1 and day 2) that incorporated researcher, clinician, and patient viewpoints. The NINDS Clinical Director, Dr. Avi Nath, is a leader in this field and the NINDS Intramural Program has made advances in understanding the effects of SARS-CoV-2 on the brain. Interestingly, Dr. Nath's team recent report in NEJM shows multifocal breakdown of the blood brain barrier, small infarcts, microhemorrhages, inflammatory infiltrates, and microglial nodules, despite no definitive sign of the virus in the brain. We are working quickly to learn more.
Many of NINDS’ scientific conferences and workshops pivoted quickly to virtual venues. While these virtual venues are no replacement for the valued in-person interactions at scientific conferences, we learned quickly that one benefit of virtual meetings is the ability to reach many more people than we normally would. NINDS events, including the 2020 Virtual Nonprofit Forum and the Virtual BRAIN Investigators Meeting, found ways to bring together community stakeholders to connect online, learn from one another, and provide networking opportunities from the comfort of home. NINDS also contributed to the NIH Virtual Seminar on Program Funding and Grants Administration, for which over 20,000 people registered to attend. As we continue to plan these virtual meetings until we can gather safely in person, I want to take a moment to recognize the active participation from the scientific community and the many NINDS staff who worked tirelessly to make the shift from in-person to virtual meetings as seamless as possible.
Events in the past year have also caused me to reflect on the NINDS mission statement of generating knowledge to reduce the burden of neurological disorders, for all people. As I wrote earlier this year, we are living in a moment that can – and must – catalyze positive change as we recognize our own behaviors and attitudes, affirm our commitment to diversity, and eliminate racial bias in our community as well as in our organization. We cannot allow this pivotal moment to pass. NIH is committed to diversity, and a recent presentation at the Advisory Committee to the NIH Director demonstrated our shared commitment to making changes. I want to encourage the neuroscience community to strongly consider the funding opportunities being made available through the Faculty Institutional Recruitment for Sustainable Transformation (FIRST) Program that aims to enhance and maintain cultures of inclusive excellence and promote diversity in the research community. Here at NINDS, we are prioritizing equity and inclusion as we update our strategic plan, and as we move forward, I continue to be committed in holding myself and our Institute accountable.
As we continue a shift to online meetings and collaboration spaces, I am proud of NINDS contributions to trans-NIH Initiatives. Along with our colleagues in the NIH Pain Consortium, we recently released a renewed Centers of Excellent in Pain Education (CoEPEs) website, providing an online resource for the development, education, and distribution of pain management curricula materials. NINDS is also leading the Early Phase Pain Investigation Clinical Network (EPPIC-Net) through the NIH Helping End Addiction Long-term (HEAL) Initiative. This effort seeks to enhance the treatment of acute and chronic pain and reduce reliance on opioids by accelerating early-phase clinical trials of non-addictive treatments for pain. The first trial in EPPIC-Net will be launched in early 2021, and I am looking forward to more trials launching throughout the year. The COVID-19 pandemic is linked to a record number of deaths from drug overdoses, primarily driven by synthetic opioids, and we are hopeful that EPPIC-Net trials will be able to lay the groundwork for pain treatments that are not reliant on opioids.
In 2020, NINDS continued to support investigators who – even in the midst of a pandemic – led innovative scientific advances in neuroscience and neurology. While I cannot highlight all of them here, I have selected a few that you can view above. This year also brought us opportunities to explore and build new research programs, collaborations, and partnerships. All of our efforts at NINDS move forward (in no small part), thanks to our leadership team. In May, Dr. John Ngai, formerly the Coates Family Professor of Neuroscience at the University of California, Berkeley, joined us as Director of the NIH BRAIN Initiative. In this role, he oversees the long-term strategy and day-to-day operations of the Initiative as it takes on the challenges of the next five-year plan. Dr. Ngai has hit the ground running, and the BRAIN Initiative continues to innovate as we accelerate the launch of large-scale transformative projects that will propel neuroscience far into the future. We anticipate that the next phase of BRAIN will take the advances the scientific community has made and move it to a greater number of researchers and into the clinic.
NINDS and the scientific community are poised for more progress in 2021, both in our ability to address the effects of the COVID-19 pandemic and in our research priorities. The year will begin with a 2.81% increase in the NINDS appropriated budget, and we continue our important mission: seeking fundamental knowledge about the nervous system and using that knowledge to reduce the burden of neurological disease, for all people.










