Department of Health and Human Services
National Institutes of Health
National Institute of Neurological Disorders and Stroke
Table of Contents
(Skip)
- Organization Chart
- Appropriation Language
- Amounts Available for Obligation
- Budget Mechanism Table
- Major Changes in Budget Request
- Summary of Changes
- Budget Graphs
- Budget Authority by Activity
- Authorizing Legislation
- Appropriations History
- Justification of Budget Request
- Budget Authority by Object Class
- Salaries and Expenses
- Detail of Full-Time Equivalent Employment (FTE)
- Detail of Positions
Organization Chart
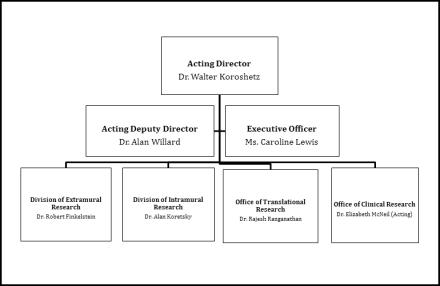
National Institutes of Health
National Institute of Neurological Disorders and Stroke
For carrying out section 301 and title IV of the PHS Act with respect to neurological disorders and stroke, [$1,605,205,000] $1,660,375,000.
Amounts Available for Obligation 1
(Dollars in Thousands)
| Source of Funding | F Y 2014 Actual |
F Y 2015 Enacted | F Y 2016 President's Budget |
|---|---|---|---|
| Appropriation | $1,587,982 | $1,605,205 | $1,660,375 |
| Type 1 Diabetes | 0 | 0 | 0 |
| Rescission | 0 | 0 | 0 |
| Sequestration | 0 | 0 | 0 |
| FY 2014 First Secretary's Transfer | -3,986 | 0 | 0 |
| FY 2014 Second Secretary's Transfer | -311 | 0 | 0 |
| Subtotal, adjusted appropriation | $1,583,685 | $1,605,205 | $1,660,375 |
| OAR HIV/AIDS Transfers | 0 | -598 | 0 |
| National Children's Study Transfers | 5,219 | 0 | 0 |
| Subtotal, adjusted budget authority | $1,588,904 | $1,604,607 | $1,660,375 |
| Unobligated balance, start of year | 0 | 0 | 0 |
| Unobligated balance, end of year | 0 | 0 | 0 |
| Subtotal, adjusted budget authority | $1,588,904 | $1,604,6077 | $1,660,375 |
| Unobligated balance lapsing | -5 | 0 | 0 |
| Total obligations | $1,588,899 | $1,604,607 | $1,660,375 |
1 Excludes the following amounts for reimbursable activities carried out by this account:
FY 2014 - $12,080 FY 2015 - $12,282 FY 2016 - $12,482
Budget Mechanism - Total 1
(Dollars in Thousands)
| MECHANISM | FY 2014 Actual |
FY 2015 Enacted | FY 2016 President's Budget |
FY 2016 +/- FY 2015 |
||||
|---|---|---|---|---|---|---|---|---|
| No. | Amount | No. | Amount | No. | Amount | No. | Amount | |
| Research Projects: | ||||||||
| Noncompeting | 1,878 | $766,564 | 1,886 | $777,277 | 1,851 | $771,799 | -35 | -$5,478 |
| Administrative Supplements | (93) | 8,074 | (111) | 10,000 | (111) | 10,000 | (0) | 0 |
| Competing: | ||||||||
| Renewal | 154 | 72,553 | 154 | 72,553 | 184 | 72,996 | 30 | 443 |
| New | 595 | 224,587 | 570 | 214,695 | 681 | 269,977 | 111 | 55,282 |
| Supplements | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Subtotal, Competing | 749 | $297,140 | 724 | $287,248 | 865 | $342,973 | 141 | $55,725 |
| Subtotal, RPGs | 2,627 | $1,071,777 | 2,610 | $1,074,525 | 2,716 | $1,24,772 | 106 | $50,247 |
| SBIR/STTR | 93 | 43,687 | 95 | 45,733 | 93 | 49,198 | -2 | 3,465 |
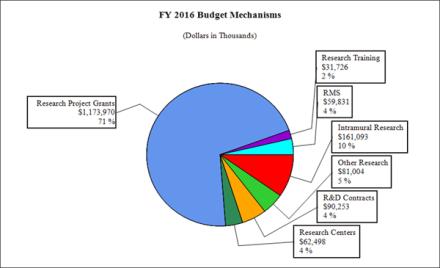
| Research Project Grants | 2,720 | $1,115,464 | 2,705 | $1,120,258 | 2,809 | $1,173,970 | 104 | $53,712 |
Research Centers: |
||||||||
| Specialized/Comprehensive | 64 | $61,273 | 65 | $62,498 | 65 | $62,498 | 0 | 0 |
| Clinical Research | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Biotechnology | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Comparative Medicine | 0 | 684 | 0 | 600 | 0 | 0 | 0 | -600 |
| Research Centers in Minority Institutions | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Research Centers | 64 | $61,957 | 65 | $63,098 | 65 | $62,498 | 0 | -$600 |
Other Research: |
||||||||
| Research Careers | 205 | $34,525 | 205 | $34,525 | 189 | $34,525 | -16 | $0 |
| Cancer Education | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cooperative Clinical Research | 83 | 21,996 | 83 | 22,216 | 93 | 22,216 | 10 | 0 |
| Biomedical Research Support | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Minority Biomedical Research Support | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 113 | 22,057 | 124 | 24,263 | 124 | 24,263 | 0 | 0 |
| Other Research | 401 | $78,578 | 412 | $81,004 | 406 | $81,004 | -6 | $0 |
| Total Research Grants | 3,185 | $1,255,998 | 3,182 | $1,264,360 | 3,280 | $1,317,472 | 98 | $53,112 |
Ruth L. Kirschstein Training Awards: |
FTTPs |
FTTPs |
FTTPs |
|||||
| Individual Awards | 383 | 15,605 | 383 | $15,839 | 383 | $16,077 | 0 | $238 |
| Institutional Awards | 324 | 15,832 | 324 | 15,418 | 324 | 15,649 | 0 | 231 |
| Total Research Training | 707 | $31,437 | 743 | $32,136 | 743 | $31,257 | 0 | $469 |
Research & Development Contracts |
97 |
$84,898 |
100 |
$90,253 |
100 |
$90,253 |
0 |
$0 |
| SBIR/STTR (non-add) | (2) | (218) | (2) | (218) | (0) | (0) | (-2) | (-218) |
| Intramural Research | 335 | 157,919 | 337 | $159,498 | 337 | 161,093 | 0 | 1,595 |
| Research Management and Support |
197 | 58,652 | 198 | 59,239 | 198 | 59,831 | 0 | 592 |
| Res. Management & Support (SBIR Admin) (non-add) | (0) | (220) | (0) | (220) | (0) | (612) | (0) | (-220) |
| Construction | 0 | 0 | 0 | 0 | ||||
| Buildings and Facilities | 0 | 0 | 0 | 0 | ||||
| Total, NINDS | 532 | $1,588,904 | 535 | $1,604,607 | 535 | $1,660,375 | 0 | $55,768 |
1 All items in italics and brackets are non-add entries.
2 The amounts in the FY 2014 column take into account funding reallocations, and therefore may not add to the total budget authority reflected herein.
Major Changes in the Fiscal Year 2016 President's Budget Request
Research Project Grants (+$53.712 million, total $1,173.970 million):
NINDS will support a total of 2,716 Research Project Grant (RPG) awards in FY 2016. Noncompeting RPGs will decrease by 35 awards $5.478 million. Competing RPGs will increase by 141 awards and $55.725 million.
Research Training (+$0.469 million, total $31.726 million):
NIH will provide an increase of two percent over FY 2015 for stipend levels for pre-doctoral and post-doctoral trainees. The requested increase will help sustain the development of a highly qualified biomedical research workforce.
Summary of Changes 1
(Dollars in Thousands)
| F Y 2015 Enacted | $1,604,607 |
|---|---|
| F Y 2016 President's Budget | $1,660,375 |
| Net change | $55,768 |
| 2016 President's Budget |
Change from F Y 2015 |
|||
|---|---|---|---|---|
| CHANGES | FTE's | Budget Authority |
FTE's | Budget Authority |
| A. Built-in: 1. Intramural research: |
||||
| a. Annualization of January 2015 pay increase & benefits |
$53,379 | $133 | ||
| b. January FY 2016 pay increase & benefits | 53,379 | 400 | ||
| c. One more day of pay (n/a for 2015) | 53,379 | 205 | ||
| d. Differences attributable to change in FTE | 53,379 | 0 | ||
| e. Payment for centrally furnished services | 26,663 | 650 | ||
| f. Increased cost of laboratory supplies, materials, other expenses, and non-recurring costs |
83,143 | 655 | ||
| Subtotal | $2,044 | |||
2. Research Management and Support: |
||||
| a. Annualization of January 2015 pay increase & benefits |
$29,294 | $73 | ||
| b. January FY 2016 pay increase & benefits |
29,294 | 220 | ||
| c. Zero more days of pay (n/a for 2015) | 29,294 | 0 | ||
| d. Differences attributable to change in FTE | 29,294 | 0 | ||
| e. Payment for centrally furnished services | 7,372 | 120 | ||
| f. Increased cost of laboratory supplies, materials, other expenses, and non-recurring costs |
23,682 | 176 | ||
| Subtotal | $761 | |||
Subtotal, Built-in |
$2804 | |||
| 2016 President's Budget |
Change from F Y 2015 |
|||
|---|---|---|---|---|
| CHANGES | No. | Amount | No. | Amount |
| B. Program: | ||||
| 1. Research Project Grants: | ||||
| a. Noncompeting | 1,851 | $781,799 | -35 | -$5,478 |
| b. Competing | 865 | 342,973 | 141 | 55,725 |
| c. SBIR/STTR | 93 | 49,198 | -2 | 3,465 |
| Subtotal, RPGs | 2,809 | $1,173,970 | 104 | $53,712 |
| 2. Research Centers | 65 | $62,498 | 0 | -$600 |
| 3. Other Research | 406 | 81,004 | -6 | 0 |
| 4. Research Training | 707 | 31,726 | 0 | 469 |
| 5. Research and Development Contracts | 100 | 90,253 | 0 | 0 |
| Subtotal, Extramural | $1,439,451 | $53,581 | ||
| FTE's | FTE's | |||
| 6. Intramural Research | 337 | $157,633 | 0 | -$449 |
| 7. Research Management and Support | 198 | 58,157 | 0 | -169 |
| 8. Construction | 0 | 0 | ||
| 9. Buildings and Facilities | 0 | 0 | ||
| Subtotal, Program | 535 | $1,642,619 | 0 | $52,964 |
| Total Changes | $55,768 | |||
1The amounts in the Change from FY 2015 column take into account funding reallocations, and therefore may not add to the net change reflected herein.
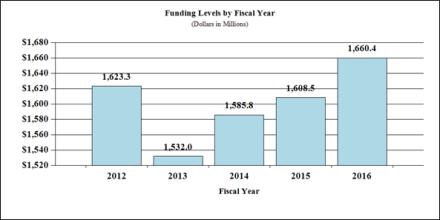
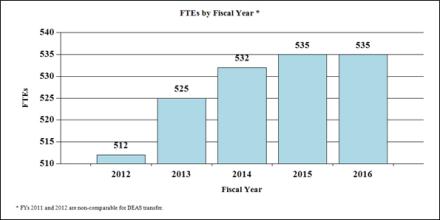
Fiscal Year 2016 Budget Graphs
History of Budget Authority and FTE's:
Distribution by Mechanism:
Change by Selected Mechanisms:
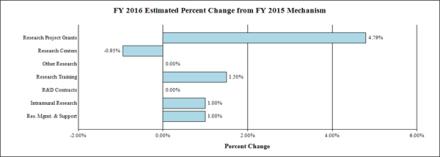
Budget Authority by Activity 1
(Dollars in Thousands)
| FY 2014 Actual |
FY 2015 Enacted |
FY 2016 President's Budget |
FY 2016 +/- FY 2015 |
|||||
|---|---|---|---|---|---|---|---|---|
| Extramural Research Detail: |
FTEs | Amount | FTEs | Amount | FTEs | Amount | FTEs | Amount |
| Channels, Synapses & Circuits | 134,347 | 135,731 | 140,979 | 5,248 | ||||
| Infrastructure, Training Programs, and Resources | 192,863 | 194,756 | 202,286 | 7,530 | ||||
| Neural Environment | 211,182 | 213,255 | 221,500 | 8,245 | ||||
| Neurodegeneration | 214,722 | 216,830 | 225,213 | 8,383 | ||||
| Neurogenetics | 193,869 | 195,772 | 203,341 | 7,569 | ||||
| Repair & Plasticity | 135,973 | 137,308 | 142,616 | 5,309 | ||||
| Systems & Cognitive Neuroscience | 231,441 | 233,713 | 242,749 | 9,036 | ||||
| Translational Research | 57,935 | 58,503 | 60,765 | 2,262 | ||||
| Subtotal, Extramural | $1,372,333 | $1,385,870 | $1,439,451 | $53,581 | ||||
Intramural Research |
335 | $157,919 | 337 | $159,498 | 337 | $161,093 | 0 | $1,595 |
Research Management & Support |
197 | $58,652 | 198 | $59,239 | 198 | $59,831 | 0 | $592 |
TOTAL |
532 | $1,588,904 | 535 | $1,604,607 | 535 | $1,604,607 | 0 | $55,768 |
1 Includes F T Es whose payroll obligations are supported by the NIH Common Fund.
Authorizing Legislation
| PHS Act/ Other Citation |
U.S. Code Citation |
2015 Amount Authorized |
F Y 2015 Enacted |
2015 Amount Authorized |
F Y 2016 PB |
|
|---|---|---|---|---|---|---|
| Research and Investigation | Section 301 | 42§241 | Indefinite | $1,604,607,000 | Indefinite | $1,660,375,000 |
| National Institute of Neurological Disorders and Stroke |
Section 401(a) | 42§281 | Indefinite | Indefinite | ||
| Total, Budget Authority | $1,604,607,000 | $1,660,375,000 |
Authorizing Legislation
| Fiscal Year | Budget Estimate to Congress | House Allowance | Senate Allowance | Appropriation |
|---|---|---|---|---|
| 2006 | $1,550,260,000 | $1,550,260,000 | $1,591,924,000 | $1,550,260,000 |
| Rescission | ($1,503,000) | |||
| 2007 | $1,524,750,000 | $1,524,750,000 | $1,537,703,000 | $1,534,757,000 |
| Rescission | $0 | |||
| 2008 | $1,537,019,000 | $1,559,106,000 | $1,573,268,000 | $1,571,353,000 |
| Rescission | ($27,452,000) | |||
| 2009 | $1,545,397,000 | $1,598,521,000 | $1,588,405,000 | $1,593,344,000 |
| Rescission | $0 | |||
| Supplemental | $8,212,000 | |||
| 2010 | $1,612,745,000 | $1,650,253,000 | $1,620,494,000 | $1,636,371,000 |
| Rescission | $0 | |||
| 2011 | $1,681,333,000 | $1,678,696,000 | $1,636,371,000 | |
| Rescission | ($14,368,312) | |||
| 2012 | $1,664,253,000 | $1,664,253,000 | $1,603,741,000 | $1,629,445,000 |
| Rescission | ($3,079,651) | |||
| 2013 | $1,624,707,000 | $1,629,631,000 | $1,626,365,349 | |
| Rescission | ($3,252,731) | |||
| Sequestration | ($81,632,357) | |||
| 2014 | $1,624,619,000 | $1,631,703,000 | $1,587,982,000 | |
| Rescission | $0 | |||
| 2015 | $1,608,461,000 | $1,605,205,000 | ||
| Rescission | $0 | |||
| 2016 | $1,660,375,000 |
Justification of Budget Request
National Institute of Neurological Disorders and Stroke
Authorizing Legislation: Section 301 and Title IV of the Public Health Service Act, as amended.
Budget Authority (BA):
| FY 2014 Actual |
FY 2015 Enacted | FY 2016 President's Budget |
FY 2016 +/- FY 2015 |
|
|---|---|---|---|---|
| BA | $1,588,904,110 | $1,604,607,000 | $1,660,375,000 | +$55,768,000 |
| F T E | 532 | 535 | 535 | 0 |
Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
Director's Overview
The National Institute of Neurological Disorders and Stroke (NINDS) supports research to improve fundamental understanding of the nervous system and to harness that knowledge to reduce the burden of neurological disorders. Statistics illustrate the magnitude of that burden. Traumatic brain injury (TBI) is the leading cause of death and disability in children and young adults, and millions of student athletes and military personnel are at risk for concussions (mild TBI), raising concern about lingering or long-delayed consequences . Strokes strike 800,000 people in the United States each year, and 6.8 million survivors live with long-term disability. Epilepsy affects nearly 1 percent of the population, 1 in 26 people at some point in life, and sudden unexpected death is 24 times more common in those who have epilepsy. Chronic pain is the most common reason people seek medical care – migraine alone affects 18 percent of women. Hundreds of neurological disorders, common and rare, present formidable challenges to medicine that are compounded by the complexity of the brain. And risk rises with age for many of these diseases, a sobering fact as aging population demographics loom.
Scientists and physicians in academia and industry agree that basic research drives long-term progress against disease. However, the financial return on basic research investment is delayed and hard to capture, so NINDS support is crucial. In 2014, for example: a Lasker Award recognized how basic research on brain control of movement led decades later to deep brain stimulation therapy, which dramatically reduces symptoms for some people with Parkinson’s disease; a new type of drug approved for insomnia built on basic studies of narcolepsy genes in dogs that identified a key molecule called orexin; and, as a direct result of NINDS research, pharmaceutical companies have initiated trials for previously neglected disorders, including brain edema in TBI, Duchenne muscular dystrophy, and spinal muscular atrophy.
New opportunities arising from basic research have naturally brought many basic scientists to study disease. However, lack of progress against disease often stems from gaps in knowledge of basic biology. NINDS is concerned about a decline over several years in the number of grant applications in basic neuroscience. To forestall an imbalance in the Institute’s research portfolio, NINDS has reaffirmed to the scientific community the Institute’s commitment to fundamental basic research through outreach efforts and has solicited proposals with set-aside funds. Reinforcing this emphasis, NINDS is an enthusiastic partner in the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) initiative, which is basic research of the most fundamental kind – to reveal how brain circuits enable us to perceive, think, and act.
NINDS-supported investigators also tackle translational and applied research that is important for patients but may not be profitable for industry. For example, commercial interest in stroke is very low compared to its enormous societal impact. NINDS’ development of an emergency treatment (tPA) that restores blood flow to the brain revolutionized stroke care, and virtually every year NINDS clinical trials advance stroke prevention. In 2014, a trial showed that monthly blood transfusions can reduce the “silent” (undetected but damaging) strokes that occur in one third of children with sickle cell anemia. The cumulative effect of many stroke advances has been substantial – a recent report found that first-time strokes declined 24 percent overall in each of the last two decades and deaths declined 20 percent per decade. A new NINDS Stroke Trials Network launched in FY 2014 will determine more efficiently what treatment, prevention, and rehabilitation strategies work best. The network builds on transformative innovations for efficiency and effectiveness in the Network for Excellence in Neuroscience Clinical Trials (NeuroNEXT), which expedites clinical studies and early phase trials of new therapies for neurological disorders, including pediatric diseases. To advance therapies to readiness for clinical testing, in 2003 NINDS launched a milestone-driven translational program that is moving therapies for neurological disorders through the development pipeline. The newly enhanced program will use contract resources, expert consultants, and better staged funding to enable investigators to meet the diverse challenges of developing drugs, devices, and biologics.
NINDS supports initiatives to harness data, promote sharing, and provide common resources across the full spectrum of research. The Parkinson’s Disease Biomarkers Program (PDBP) is developing measurable indicators of Parkinson’s disease onset and progression to accelerate therapy development. In 2014, the PDBP enrolled its 1000th patient, released a treasure trove of data for the research community, and received a prestigious government-wide award for its use of information technology. The Common Data Elements (CDE) program brings together the disease community and Federal agencies to establish data standards that foster comparison across clinical studies. The TBI CDEs provide the essential foundation for the Federal Interagency TBI Research Database (FITBIR), which holds data from NIH and the Department of Defense research. FITBIR will include data from two new, prospective, observational studies, TRACK-TBI (primarily adults) and ADAPT (children), which are coordinated with the International TBI Research Initiative, a partnership among NIH, the Canadian Institute of Health Research and the European Union. The goal is to determine which therapies work best for each person. Other resources for research include the NeuroBIOBANK, which is a coordinated approach to brain banking, the Human Connectome Project, with images of neural pathways that underlie function in the living brain, and extensive programs for human genetics and induced pluripotent stem cells. The torrent of multi-dimensional data from the BRAIN Initiative will spur innovation in informatics and computational science that is likely to spillover far beyond neuroscience.
NINDS has many programs and policies to sustain a diverse and talented workforce, which is the most essential requirement for progress. The Pathway to Independence Awards and a more generous payline for early stage investigators provide young scientists a fair chance in the highly competitive funding environment. Career development programs address special problems of physician scientists, including neurosurgeons, who pursue research. A NINDS-wide diversity working group actively engages all the Institute’s programs to recruit a diverse scientific workforce across all career stages. Neuroscience benefits greatly from NIH programs to foster innovative researchers, including New Innovator, Pioneer, and Transformative R01 Awards. The Javitz Neuroscience Investigator Awards have long recognized the value of long-term support for exceptional researchers, and a new grant program will also enable talented investigators to direct more creativity to doing research and less time to obtaining funding. Attracting talented researchers and providing support for them to do their best research is, simply put, the highest priority NINDS must address to fulfil its mission. Perhaps the most important legacy of the BRAIN Initiative may be engaging the next generation of scientists in unravelling the mysteries of the brain and its ailments.
Program Descriptions and Accomplishments:
Overall Budget Policy: The FY 2016 President’s Budget request is $1,660.375 million, an increase of $55.768 million or 3.475 percent, over the FY 2015 Enacted level. NINDS emphasizes investigator-initiated research throughout its basic, translational, and clinical programs. Complementing these programs, the Institute solicits research proposals to address unmet mission-critical scientific opportunities, address public health needs, and provide common resources to enhance sharing and catalyze productivity. To sustain funding success rates, encourage new investigators, and launch new mission critical programs within the level of resources available, NINDS rigorously evaluates programs, in consultation with the National Advisory Neurological Disorders and Stroke Advisory Council and outside experts, and closes legacy projects that no longer warrant investment, because goals have been met, science has advanced, or programs have not proven effective. By scheduling interim analyses of large clinical trials and monitoring milestones in expensive preclinical therapy development programs, NINDS can also shift funds to the best current opportunities. The Institute scrutinizes the mission priority of all requests to submit applications for large investigator-initiated projects and sunsets multi-investigator program project grants after one renewal period. Across all programs, NINDS has enhanced attention to transparency in reporting of research and to rigor and reproducibility of research, especially for studies that justify major investments in preclinical therapy development or clinical trials. NINDS leadership on this issue has led to significant changes within NIH, scientific journals, and the scientific community. In FY 2014 NIH launched the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) initiative as a large-scale effort to equip researchers with fundamental insights necessary for treating a wide variety of devastating brain disorders like Alzheimer’s, schizophrenia, autism, epilepsy, and traumatic brain injury. NIH is requesting a total of $70 million in new funding for this Presidential initiative. As one of the leaders of the BRAIN, NINDS is requesting an increase of $23 million in its budget to support this priority.
1Circulation 1 34:e6-245, 2013
Channels, Synapses, and Circuits:
Ion channels, synapses, and circuits of nerve cells are fundamental components of the nervous system. Ion channels control electrical currents in cells. Synapses are the specialized cell-to-cell connections by which nerve cells influence the activity of other nerve cells. Circuits formed by networks of precisely interconnected nerve cells carry out the information processing that enables us to perceive, think, and act. Decades of progress, recognized by several Nobel Prizes, has revealed in remarkable detail the molecular machinery that controls ion channels and synapses, and brain imaging has brought big picture insights about the functions of whole brain regions. Until recently, however, technology has limited the ability of researchers to study what occurs in the brain between these two extremes, that is, how large numbers of interconnected nerve cells form circuits that underlie perception, thinking, memory, and movement. Long-term investments in basic science research have now laid groundwork that makes possible the BRAIN Initiative, which is taking on this grand challenge of developing powerful new tools to understanding how brain circuits work. The BRAIN Initiative promises to transform virtually all areas of NINDS research. Epilepsy, which is a major focus of the Channels, Synapses, and Neural Circuits Program, arises from abnormal excitability of the brain and will ultimately benefit from better understanding of brain circuits. Since 2001, the Epilepsy Benchmarks process has brought NINDS, the research community, and non-governmental organizations together to establish milestones and monitor progress toward the goal of “no seizures, no side effects.” The April 2013 conference Curing the Epilepsies 2013: Pathways Forward assessed progress on the Epilepsy Benchmarks and led to the 2014 NINDS Benchmarks for Epilepsy Research, which will guide the Institute and the scientific community in 2015 and beyond.
Budget Policy:
The FY 2016 President’s Budget request is 140.979 million, an increase of $5.248 million or 3.86 percent, over the FY 2015 Enacted level. In FY 2016, NINDS will continue to balance investigator-initiated research and research targeted to specific mission priorities, including projects funded through the Institute’s translational research and clinical trials programs. Among the targeted initiatives, the Epilepsy Centers without Walls bring together the best multidisciplinary team of investigators, regardless of geographic locations, to focus for multiple years on a specific Benchmarks priority that is better addressed through such a cooperative effort rather than traditional grant mechanisms. NINDS and NHLBI have also collaborated to expand the CDC’s Sudden Unexpected Infant Death (SUID) Case Registry to include SUDEP and Sudden Cardiac Death in individuals up to age 24 in 15 states. NINDS is also a lead Institute in the development and implementation of the BRAIN Initiative. NINDS is working closely with other parts of NIH, other Federal agencies, and private groups to implement the scientific vision that was developed by an interdisciplinary team of scientists after extensive consultation with the scientific community. To that end, BRAIN Initiative notices of funding opportunities have been issued for FY 2014, FY 2015, and will be issued for FY 2016.
Program Portrait: Epilepsy Centers Without Walls
FY 2015 Level: $4.5 million
FY 2016 Level: $5.0 million
Change: + $0.5 million
In 2011, NINDS launched the Epilepsy Centers without Walls (CWOW) program to take on key priorities of the Epilepsy Benchmarks that are not adequately addressed though investigator-initiated research and other existing programs. Each CWOW brings together the best possible team of investigators to tackle an important problem in epilepsy research. The first CWOW recognized that a cooperative application of new genetics methods to discover genes that cause epilepsy would be more effective than individual laboratories working alone. That CWOW, which built on earlier NINDS programs in epilepsy genetics, has already identified new genes for infantile spasms and Lennox-Gastaut Syndrome (severe types of childhood epilepsy), found evidence of several other epilepsy-related genes, developed powerful new analytic methods, and established a collaboration with Citizens United for Research in Epilepsy (CURE) to create a data repository that will help patients and advance research.1 The second CWOW targets sudden unexplained death in epilepsy (SUDEP), which for unknown reasons occurs 24 times more often in people with epilepsy than in the general population. Because no teams were ready to take on this challenge, NINDS first held a scientific workshop on SUDEP, then solicited and supported exploratory grants to allow research groups to come together, and, after a follow-up solicitation, brought together the best team in a CWOW in FY 2014.
In FY 2015 and 2016 NINDS is expanding the CWOW program with a new focus on preclinical and clinical research to develop therapies that prevent epilepsy or attenuate the disease process itself. Drugs and other treatments do not control seizures in about one third of people with epilepsy. Even people whose seizures are controlled must contend with difficult side effects, including depression, anxiety, cognitive issues, and developmental delays that may seriously diminish quality of life. Developing therapies that address the underlying causes of epilepsy or prevent its development, rather than merely suppressing seizures, is one of the highest priorities of the Epilepsy Benchmarks.
1Nature 501:217-21,2013
Infrastructure, Training programs, and Resources:
The Office of International Activities (OIA), Office of Training, Career Development, and Workforce Diversity, and Office of Clinical Research coordinate activities that span the breadth of NINDS, working with the relevant scientific and disease experts across Institute programs. OIA leads international collaborations that advance the NINDS mission and coordinates the Institute’s global health activities, working closely with the Fogarty International Center. Following the advice of planning panels on workforce diversity, the Institute integrated its programs on diversity within the Office of Training, Career Development, and Workforce Diversity, which coordinates extensive NINDS extramural programs for training, career development, and workforce diversity, including programs tailored to the specific needs of physician-scientists. In 2014, the Society for Neuroscience, the world's largest professional organization for neuroscience, received a major award for the NINDS-supported Neuroscience Scholars Program, which enhances the professional and career progress of underrepresented and diverse graduate and post-doctoral neuroscientists. The Office of Clinical Research (OCR) supports infrastructure and programs for clinical research, including early and advanced phase clinical trials. On advice of a strategic planning panel on health disparities, the Institute integrated health disparities research within OCR, including large epidemiological studies, and convened a multi-agency, multi-stakeholder conference to develop plans for addressing disparities in stroke mortality and morbidity. Over the last several years, OCR has significantly increased the efficiency and effectiveness of clinical trials, which are inherently expensive, by setting milestones for progress, providing resources to improve patient access and recruitment, and developing multi-site clinical networks that provide common resources and economies of scale. The Network for Excellence in Neuroscience Clinical Trials (NeuroNEXT), which carries out clinical studies and early phase clinical trials of novel therapies for neurological disorders pioneered use of a single Institutional Review Board and master agreements among clinical sites that substantially reduce the time and cost required to launch new trials. Building on the innovations from NeuroNEXT, a new Stroke Clinical Trials Network, will conduct clinical trials for prevention, treatment, and rehabilitation of stroke more quickly and for less cost. The OCR Common Data Elements (CDE) program works with the research and patient advocacy communities to facilitate comparison and sharing of clinical data across studies, enhancing the value of these major clinical investments.
Budget Policy: The FY 2016 President’s Budget request is $202.286 million, an increase of $7.530 million or 3.86 percent, over the FY 2015 Enacted level. The NeuroNEXT clinical network is fully operational, with four major clinical studies underway through central data and clinical coordinating centers and 25 clinical sites across the U.S. In 2016, NINDS will continue to solicit proposals for NeuroNEXT pediatric and adult clinical studies from academic investigators, foundations, small businesses, and industry. The new Stroke Trials Network enhances the effectiveness of small and large stroke clinical trials by eliminating the costs and time associated with developing the infrastructure for each major trial anew and incorporating other efficiencies, such as central Institutional Review Boards and master contract agreements. The Network established regional coordinating centers, a national clinical coordinating center, a national data management center, and clinical sites, and began activities in FY 2014 by enhancing ongoing NINDS multi-center stroke trials. In FY 2015, the first new projects will move forward in the network and in FY 2016 the Institute will continue to seek clinical trial proposals for the Stroke Network from academic, industry, and small business investigators. The Neurological Emergency Treatment Trials Network (NETT) is currently undergoing an external review that will report to the National Advisory Neurological Disorders and Stroke Council (NANDS) Council in January 2015. NINDS has been in discussions with NHLBI about initiating an emergency trial network that could answer important clinical questions for multiple NIH ICs as well as trauma research of importance to the Department of Defense. NHLBI Council has approved the initiative and the NINDS council will review the proposal at its January Council meeting. NINDS is also continuing its support for phase III investigator-initiated clinical trials across all neurological disorders. Because hypertension is the largest cause of stroke, and stroke disparities persist despite progress, in FY 2015 NINDS and NHLBI are partnering with the Patient Centered Outcomes Research Institute (PCORI) to solicit proposals to study how to improve blood pressure control in high-risk individuals, including racial and ethnic minorities. A full range of NINDS programs in training and career development are also continuing, including individual and institutional grants at the graduate, post-doctoral, and career development levels, and an intensive training course in clinical trials methods for fellows and faculty in the clinical neurosciences. Many of the programs target special needs, such as promoting diversity and enabling neurosurgeons to accommodate research preparation into their demanding training requirements.
Neural Environment:
Non-nerve cells, called glial cells, outnumber nerve cells in the brain. Glial cells, together with specialized blood vessels and immune cells in the brain, maintain the local environment around nerve cells, fight infections, control which molecules enter brain tissue from the circulating blood, and actively shape brain development and synapse function. Neurological disorders can result when non-neuronal cells are compromised; when these cells become aggressors in inflammatory or autoimmune disorders; when cells form tumors; when viruses, bacteria, or parasites infect the nervous system; or when the blood supply to brain cells is compromised. The Neural Environment Program supports basic research on the neural environment, on diseases in which its disruption plays a major role, and on translation of basic scientific knowledge into diagnostic tools, preventive measures, and targeted therapies. Stroke is a major focus, and the Neural Environment Program coordinates NINDS’ stroke activities, which span several parts of the Institute and have had a major impact on public health. Multiple sclerosis is another common disorder that affects glial cells. Basic research on immunology of the brain and glial cells has fostered private sector development of more than 10 drugs for multiple sclerosis over the last two decades and hospitalization rates due to multiple sclerosis have decline by 75 percent over that period. Many recent findings from basic research on the neural environment may have an impact in the future. For example, researchers have identified glial cell-controlled conduits for cerebral spinal fluid flow between brain cells that allow the brain to flush out toxins, and other researchers demonstrated that circulating factors in the blood of young mice can counteract some of the negative effects of aging on the brains of older mice via effects on the brain blood vessels.
Budget Policy: The FY 2016 President’s Budget request is $221.500 million, an increase of $8.245 million, or 3.8 percent, over the FY 2015 Enacted level. NINDS will continue to balance investigator-initiated research and research targeted to specific priorities, including research through the Institute’s translational research and clinical trials programs. The 2012 Stroke Research Priorities planning, the 2013 Alzheimer’s Disease-Related Dementias planning conference, and a 2014 trans-NIH scientific workshop on Small Blood Vessels all emphasized the importance of brain blood vessel health. NIH is supporting increasing research in this area, which is crucial for stroke, Alzheimer’s disease, and other forms of neurodegeneration, including a FY 2015 NIA-NINDS solicitation on Vascular Contributions to Alzheimer’s Disease.
Neurodegeneration:
The Neurodegeneration program focuses on adult onset neurodegenerative diseases, that is, diseases in which brain cells progressively die. Alzheimer's disease, amyotrophic lateral sclerosis (ALS, or Lou Gehrig’s disease), frontotemporal dementias (FTD), Huntington’s disease, Parkinson’s disease, and vascular cognitive impairment are among the neurodegenerative diseases that affect adults. The program supports research to understand these diseases and to translate basic findings into clinical practice. Shared mechanisms that contribute to multiple neurodegenerative disorders present a major opportunity for progress. Research has revealed, for example, that proteins form abnormal aggregates in several of these diseases that may propagate from one brain cell to another, revealing a target for intervention. Over the last several years, the identification of genes that can cause Alzheimer’s disease, Parkinson’s disease, ALS, frontotemporal dementia, and other neurodegenerative diseases has driven progress for both inherited and non-inherited neurodegenerative diseases, because biological pathways revealed by gene identification are often affected in non-inherited diseases as well. In 2012 and 2013 separate expert planning panels on stroke and on Alzheimer’s Disease Related Dementias emphasized the importance of attending to the strong connections between brain blood vessels and dementias, including Alzheimer’s. These interrelationships are becoming apparent at every level of research from molecular mechanisms to behavioral risk factors. NINDS is working with other parts of NIH on this issue. Progress in preventing dementia may benefit from the considerable success in preventing stroke – indeed recent observations reported at an international conference suggest that in the United States and in Europe rates of dementia may indeed be falling and onset coming later.5
Budget Policy: The FY 2016 President’s Budget request is $225.213 million, an increase of $8.383 million or 3.86 percent, over the FY 2015 Enacted level. NINDS neurodegeneration research will continue to balance investigator-initiated research and solicited research, including therapy development funded through the Institute’s translational research and clinical trials programs. In keeping with the Institute’s commitment to periodic strategic planning for Parkinson’s disease, a January 2014, NINDS workshop engaged the scientific community and the public in identifying basic, translational, and clinical priorities for Parkinson’s disease research. The plan was presented to the NANDS Council in February 2014 and will guide Parkinson’s research activities. Among the major activities, in FY 2015 NINDS is reissuing the notice of funding opportunity for the Morris K. Udall Parkinson’s Disease Centers of Excellence program. This program supports a network of centers that work independently and collaboratively, sharing resources and data with the scientific community, to define the causes of and discover improved treatments for Parkinson’s disease. In 2014 the Parkinson’s Disease Biomarkers Program recruited its 1000th subject, marking a major milestone in the effort to develop methods to predict the early onset and track the progression of Parkinson’s disease. The Program’s data management resource was cited as the Overall Winner for 2014 in the Excellence.gov Awards, from a public-private partnership panel honoring the best of government information technology. A solicitation for Parkinson’s Disease Biomarker Program Discovery Projects is continuing in FY 2016. The recommendations from a 2013 NINDS-led cientific conference on Alzheimer’s Disease Related Dementias are now an integral component of the National Alzheimer’s Project Act (NAPA) plan that is guiding further research in this area.
Neurogenetics:
Gene defects cause hundreds of rare diseases that affect the nervous system, including ataxias, Down syndrome, dystonias, fragile X syndrome, lysosomal storage diseases, muscular dystrophies, peripheral neuropathies, Rett syndrome, spinal muscular atrophy, Tourette syndrome, and tuberous sclerosis, among many others. Genes also influence susceptibility to common neurological disorders. The Neurogenetics Program supports research on genes that cause or influence neurological disorders, molecular mechanisms through which these genes act, genetic animal and cell models of human disease, and development of treatments for neurogenetic disorders, from the laboratory to the clinic. Basic research to understand how genes and the environment orchestrate brain development is also a key aspect of the neurogenetics program. Over the last two decades, research has identified hundreds of genes related to neurological disorders, which has led to tests that enable physicians to diagnose a disease months or even years more quickly than before. Gene findings have also led to animal models that mimic key aspects of human diseases, insights about disease mechanisms, and rational strategies for therapy development. Therapies now emerging from this pipeline into clinical testing in the public and private sector have generated cautious optimism that the first effective treatments for spinal muscular atrophy, muscular dystrophies, Rett syndrome, and many other rare genetic diseases may be on the horizon.
Budget Policy: The FY 2016 President’s Budget request is $203.341 million, an increase of $7.569 million or 3.86 percent, over the FY 2015 Enacted level. NINDS will continue to support investigator-initiated grants and targeted activities in neurogenetics, including projects funded through the Institute’s translational research and clinical trials programs. The Institute is providing substantial support for the application of whole genome sequencing and other “next generation” genomics methods to neurological disorders. Major continuing trans-NIH programs include the Paul D. Wellstone Muscular Dystrophy Cooperative Research Centers and the Autism Centers for Excellence. In 2015, the Muscular Dystrophy Coordinating Committee will complete an update of the Action Plan for the Muscular Dystrophies, which will provide guidance not only for NIH activities in muscular dystrophy, but also for other public and private entities. NINDS will continue to work closely with the NIH Office of Rare Disease Research in supporting and providing disease specific expertise to NINDS mission relevant consortia within the Rare Diseases Clinical Research Network and the Network’s Coordinating Center. NINDS will also continue to support resources for neurogenetics research that enhance the efficiency and effectiveness of research by supporting sharing of data, biological samples, and research tools.
Program Portrait: Fundamental Neuroscience
FY 2015 Level: $5.0 million
FY 2016 Level: $5.5 million
Change: + $0.5 million
Long-term progress against neurological disease depends on a robust and balanced pipeline across the spectrum of research, from fundamental studies to understand the normal brain through research on disease mechanisms, applied preclinical development, and clinical testing of therapies. An extensive NINDS analysis found that there has been a progressive decline since 1997 in the proportion of the Institute’s budget that funds basic neuroscience research on the normal brain and nervous system. To some extent this is a healthy shift towards more disease-focused and applied research that reflects progress in neuroscience. Basic science has, after all, revealed exciting new opportunities to understand disease and presented for the first time rational targets for development of drug, device, gene, and cell therapies for many disorders. However, today’s opportunities all arose from past investments in fundamental research. NINDS has a unique responsibility to support research on which future progress in the public and private sector depends.
NINDS has taken several steps to restore the vigor of fundamental neuroscience. The Institute has worked with the Center for Scientific Review to reemphasize to peer reviewers the importance of basic science to the NIH mission. NINDS leaders have accentuated to the scientific community, and especially young scientists, the Institute’s continued commitment to fundamental research through prominent journal statements and other outreach activities. NINDS has selected especially meritorious fundamental neuroscience grants though its High Program Priority process, which funds grants in mission critical areas that score just beyond the payline. Recent data suggest that these actions have begun to reverse the decline in basic neuroscience. To further reinforce the importance of fundamental neuroscience research, NINDS has issued a program announcement with set aside funds that, beginning in FY 2015 and continuing for three years, will and support meritorious grants in any area of fundamental neuroscience within the Institute’s mission.
Repair and Plasticity:
The Repair and Plasticity Program leads NINDS research on traumatic brain injury (TBI), spinal cord injury, and peripheral nerve injury. Research covers the full spectrum from studies of mechanisms of immediate damage, through delayed effects in the hours after initial injury, to the laboratory development of interventions in animal models and clinical testing in people. The Program also supports fundamental studies of neural plasticity, that is, the ability of the brain and nervous system to change, including studies of neural stem cell biology. Neural plasticity has broad implications for recovery following injury or disease. The Repair and Plasticity Program also provides all NINDS programs with expertise in bioengineering and leads the Neural Interfaces Program, which for more than three decades has pioneered the development of devices that interface with the nervous system to restore lost functions. This program has made major contributions to the development of cochlear implants, technologies for deep brain stimulation (DBS), brain computer interfaces, and many other advances.
Budget Policy: The FY 2016 President’s Budget request is $142.616 million, an increase of $5.309 million or 3.86 percent, over the FY 2015 Enacted level. NINDS continues to balance investigator-initiated research and solicitations, including projects funded through the Institute’s translational research and clinical trials programs. Large observational studies, now underway, will provide critical information to improve clinical care for TBI and clinical trials of interventions. These studies include the Transforming Research and Clinical Knowledge in TBI (TRACK TBI) study of adults and children with TBI at 11 sites in the U.S. and the Multiple Medical Therapies for Pediatric TBI (ADAPT Trial), which will focus on 1000 children with severe TBI. Both of these studies are part of the continuing International TBI Research Initiative that was launched in FY 2014 in coordination with the European Union and the Canadian Institute of Health Research. These studies use the NINDS TBI Common Data Elements, developed with other Federal agencies and the international research community, and the NIH-Department of Defense led Federal Interagency TBI Informatics System (FITBIR) database to encourage sharing of data. With regard to the important public health issues of sports related TBI, NINDS will continue to work closely with the Foundation for NIH (fNIH) Sports and Heath Research Program. The fNIH created this program with a major donation from the National Football League. The program has launched two major cooperative projects to define the scope of long-term changes that occur in the brain years after a head injury or after multiple concussions and to improve diagnostic criteria, as well as several pilot projects. In 2016, the Neural Interfaces Program, will continue to solicit and support projects to translate advanced neural prosthetics and other devices up to and through “first in human” clinical demonstrations. NINDS supports device development through a series of notices of funding opportunities coordinated with NINDS Office of Translational Research and issued in FY 2014. The Cooperative Research to Enable and Advance Translational Enterprises – Devices (CREATE-Devices) program provides funding mechanisms tailored to the unique needs of device development at various stages and to the different FDA device approval pathways.
Systems and Cognitive Neuroscience:
Systems of interconnected nerve circuits in the brain, spinal cord, and body control learning, memory, attention, language, thinking, emotion, sensation, movement, and response to pain, as well as sleep, feeding, and drinking. The Systems and Cognitive Neuroscience Program supports research on how the brain carries out these complex functions, on their disruption in neurological disorders, and on promoting recovery. Non-invasive brain imaging is an important research tool for this program, including monitoring of brain activity associated with specific cognitive and behavioral processes. Chronic pain disorders, including migraine and other headaches, are among the most prevalent of all medical conditions and are a high priority for this program. NINDS leads NIH pain research, which is coordinated through the Office of Pain Policy and the NIH Pain Consortium. NINDS also leads the Interagency Pain Research Coordinating Committee (IPRCC), which coordinates the wider Federal and private sector communities. In May 2014, NIH and the Interagency Pain Research Coordinating Committee launched the Interagency Pain Research Portfolio, a database that provides the public and the research community information on the breadth and details of pain research and training activities supported by six federal agencies. The NINDS Office of Pain Policy manages the database.
Budget Policy: The FY 2016 President’s Budget request is $242.749 million, an increase of $9.036 million or 3.86 percent, over the FY 2015 Enacted level. NINDS balances investigator initiated research and solicitations, including projects funded through the Institute’s translational research and clinical trials programs. Pain continues to be a major area of emphasis, with NINDS coordinating NIH activities through the NIH Pain Consortium. In 2015, the NINDS Office of Pain Policy, acting through the IPRCC, will lead the development of a federal pain research portfolio long term strategy. NINDS continues to support the NIH Pain Consortium Centers of Excellence for Pain Education, which act as hubs for the development, evaluation, and distribution of pain management curriculum resources for medical, dental, nursing and pharmacy schools to enhance and improve how health care professionals are taught about pain and its treatment. Other solicitations continuing in FY 2016 focus on chronic overlapping pain conditions, on the neurobiology of migraine, and on mechanisms, models, measurement, and management in pain research.
Translational Research:
The Office of Translational Research (OTR) leads NINDS preclinical translational activities for all diseases within the Institute’s mission. OTR supports the development of drugs, devices, and biologics, including cell and gene therapies. OTR preclinical therapy development experts work closely with disease-specific experts in extramural programs across the Institute and with the Office of Clinical Research to advance these interventions through the development pipeline to first-in-human studies. NINDS launched the OTR milestone-driven preclinical therapy development program in 2003, and the program has advanced preclinical drug, gene therapy, and cell therapy projects for into further development for Parkinson’s, Batten disease, muscular dystrophy, stroke, and other disorders. Following extensive analysis, OTR has substantially revised this program. The revised program maintains the milestone-based funding and peer reviewer expertise in therapy development, but more seamlessly advances projects from early proof-of-concept through first-in-human testing, with enhanced use of expert consultants and contract resources for key steps in the development process that are outside the expertise of most academic laboratories. The new program also now tailors funding mechanisms to the needs of different therapeutic modalities. The CREATE Bio (Cooperative Research to Enable and Advance Translational Enterprises for Biotechnology Products and Biologics) program supports biotechnology product and biologics therapies, including peptides, proteins, oligonucleotides, gene therapies, and cell therapies. CREATE Devices supports device development along the different FDA-approval pathways for devices. The NIH Blueprint Neurotherapeutics Program, which has also been expanded and modified, supporst small molecule drug development, with greater flexibility to provide a mix of grant support and access to contract resources. The OTR Innovative Grants to Nurture Initial Translational Efforts (IGNITE) program supports the earlier research and development work necessary to meet the entry requirements for the CREATE and Blueprint Neurotherapeutics therapy development programs. OTR continues to coordinate all NINDS SBIR and STTR programs, working with experts in other Institute programs as appropriate.
Budget Policy: The FY 2016 President’s Budget request is $60.765 million, an increase of $2.262 million or 3.86 percent, over the FY 2015 Enacted level. This includes programs led by the Office of Translational Research, but does not include all NINDS translation research activities, which are also supported through budgets of other program areas as appropriate to the disease of focus. NINDS issued solicitations for the newly designed CREATE-BIO and CREATE-DEVICES and for the revised Blueprint Neurotherapeutics program in FY 2014, thus providing appropriate avenues for funding development of all relevant therapeutic modalities that will continue in FY 2016. In early FY 2015, the IGNITE program issued solicitations focusing on Pharmacodynamics and In vivo Efficacy Studies for Small Molecules and Biologics/Biotechnology Products and on Assay Development and Therapeutic Agent Identification and Characterization to Support Therapeutic Discovery. These and solicitations to be issued later in FY 2015 will continue in FY 2016 to support earlier phases of therapy development that are not well supported by existing programs. A panel of outside experts has been convened to report to the NANDS Council in FY 2015 on recommendations for the continuing Anticonvulsive Screening Program that will guide its activities in FY 2016. The SBIR and STTR programs are also continuing in FY 2016 under the direction of OTR.
Intramural Research:
IRP conducts research on the NIH campus in Bethesda, Maryland. The Program spans basic and translational neuroscience, neurology, and neurosurgery, and maintains its historical strengths in neuromuscular diseases, neuroimmunology, membrane biochemistry and biophysics, and neurophysiology on the system and synaptic level. The Mark O. Hatfield Clinical Center on the NIH campus in Bethesda, which is a hospital totally dedicated to clinical research, and the NIH Porter Neuroscience Research Center, which integrates neuroscience across institutes and disciplinary boundaries, provide unique resources to the Intramural Research Program. In 2014, a Blue Ribbon Panel of distinguished extramural scientists reviewed the NINDS Intramural Research Program, based on extensive data on the program and on staff interviews. In September 2014, the panel reported specific recommendations to the NANDS Council on how to build on the IRP’s strengths, leverage opportunities, and overcome challenges, with the overall goal of enhancing the IRP’s contribution to the Institute’s mission. In its report, the panel noted that IRP has outstanding leadership, leads NIH in integrating science within and across Institutes, provides outstanding support for basic science in the context of the clinical mission, and is vigilant for opportunities to strengthen and expand that clinical mission. The panel also highlighted several areas of special scientific strength ranging from basic molecular neuroscience to imaging research, and specific clinical focus areas, such as neurogenetics, neuroimmunology, neurovirology, movement disorders, stroke, and surgical neurology.
Budget Policy: The FY 2016 President’s Budget request is $161.093 million, an increase of $1.595 million or 1 percent, over the FY 2015 Enacted level. The recommendations of the Blue Ribbon Panel and the parallel development of an IRP strategic plan and long term vision will guide the IRP in 2016 as it maintains its strengths in basic and translational neuroscience and recruits exceptional physician scientists to complement its strengths in clinical neuroscience. Based upon the recommendations of the blue ribbon panel the IRP plans to seek additional physician scientists in neurogenetics, neuroimmunology and neurovirology, stroke, movement disorders, and surgical neurology. A goal will be to develop a central infrastructure to become a premier site for first-in-human trials of treatments for neurological disorders and stroke, which will require enhancing collaborations with neighboring hospitals and medical schools. The IRP will continue its collaborations through Center for Neuroscience and Regenerative Medicine (CNRM) with the Department of Defense, including the Walter Reed National Military Medical Center and the Uniformed Services University. CNRM, brings together clinicians and scientists across disciplines to catalyze innovative approaches to traumatic brain injury research.
Research Management and Support (RMS):
RMS activities provide administrative, budgetary, logistical, and scientific support in the review, award, and monitoring of research grants, training awards, and research and development contracts. RMS functions also encompass strategic planning, coordination, and evaluation of the Institute’s programs, regulatory compliance, international coordination, and liaison with other Federal agencies, Congress, and the public.
Budget Policy: The FY 2016 President’s Budget request is $59.831 million, an increase of $0.592 million or 1.0 percent over the FY 2015 Enacted level.
Budget Authority by Object Class 1
(Dollars in Thousands)
| FY 2015 Enacted |
FY 2016 President's Budget |
FY 2016 +/- FY 2015 |
|
|---|---|---|---|
| Total compensable workyears: | |||
| Full-time employment | 535 | 535 | 0 |
| Full-time equivalent of overtime and holiday hours | 0 | 0 | 0 |
| Average ES salary (in whole dollars) | $182 | $182 | $0 |
| Average GM/GS grade | 12.5 | 12.5 | 0.0 |
| Average GM/GS salary (in whole dollars) | $100 | $100 | $0 |
| Average salary, grade established by act of July 1, 1944 (42 U.S.C. 207) (in whole dollars) |
$0 | $0 | $0 |
| Average salary of ungraded positions (in whole dollars) | $0 | $0 | $0 |
| OBJECT CLASSES | FY 2015 Enacted |
FY 2016 President's Budget |
FY 2016 +/- FY 2015 |
|---|---|---|---|
| Personnel Compensation: | |||
| 11.1 Full-time permanent | $30,676 | $31,101 | $424 |
| 11.3 Other than full-time permanent | 23,074 | 23,393 | 319 |
| 11.5 Other personnel compensation | 1,437 | 1,457 | 20 |
| 11.7 Military personnel | 648 | 657 | 9 |
| 11.8 Special personnel services payments | 8,163 | 8,276 | 113 |
| Total, Personnel Compensation | $63,999 | $64,884 | $885 |
| 12.0 Personnel benefits | $17,173 | $17,344 | $586 |
| 12.2 Military personnel benefits | 438 | 444 | 6 |
| 13.0 Benefits for former personnel | 0 | 0 | 0 |
| Subtotal, Pay Costs | $81,610 | $82,673 | $1,063 |
| 21.0 Travel and transportation of persons | $3,653 | $3,784 | $63 |
| 22.0 Transportation of things | 293 | 298 | 5 |
| 23.1 Rental payments to GSA | 1 | 1 | 0 |
| 23.2 Rental payments to others | 62 | 62 | 1 |
| 23.3 Communications, utilities and miscellaneous charges | 609 | 619 | 10 |
| 24.0 Printing and reproduction | 0 | 0 | 0 |
| 25.1 Consulting services | $2,981 | $3,028 | $48 |
| 25.2 Other services | 35,076 | 34,186 | -889 |
| 25.3 Purchase of goods and services from government accounts | 140,903 | 142,956 | 2,053 |
| 25.4 Operation and maintenance of facilities | $1,840 | $1,840 | 0 |
| 25.5 Research and development contracts | 21,740 | 21,241 | -499 |
| 25.6 Medical care | 1,590 | 1,629 | 40 |
| 25.7 Operation and maintenance of equipment | 3,346 | 3,400 | 54 |
| 25.8 Subsistence and support of persons | 0 | 0 | 0 |
| 25.0 Subtotal, Other Contractual Services | $207,476 | $208,281 | $806 |
| 26.0 Supplies and materials | $8,645 | $8,783 | $138 |
| 31.0 Equipment | 6,640 | 6,746 | 106 |
| 32.0 Land and structures | 0 | 0 | 0 |
| 33.0 Investments and loans | 0 | 0 | 0 |
| 41.0 Grants, subsidies and contributions | 1,295,617 | 1,349,198 | 53,581 |
| 42.0 Insurance claims and indemnities | 0 | 0 | 0 |
| 43.0 Interest and dividends | 0 | 0 | 0 |
| 44.0 Refunds | 0 | 0 | 0 |
| Subtotal, Non-Pay Costs | $1,522,997 | $1,577,702 | $54,705 |
| Total Budget Authority by Object Class | $1,604,607 | $1,660,375 | $55,768 |
1Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
Salaries and Expenses
(Dollars in Thousands)
| OBJECT CLASSES | FY 2015 Enacted |
FY 2016 President's Budget |
FY 2016 +/- FY 2015 |
|---|---|---|---|
| Personnel Compensation: | |||
| Full-Time Permanent (11.1) | $30,676 | $31,101 | $424 |
| Other Than Full-Time Permanent (11.3) | 23,074 | 23,393 | 319 |
| Other Personnel Compensation (11.5) | 1437 | 1457 | 20 |
| Military Personnel (11.7) | 648 | 657 | 9 |
| Special Personnel Services Payments (11.8) | 8,163 | 8,276 | 113 |
| Subtotal Personnel Compensation (11.9) | $63,999 | $64,884 | $885 |
| Civilian Personnel Benefits (12.1) | $17,173 | $17,344 | $172 |
| Military Personnel Benefits (12.2) | 438 | 444 | 6 |
| Benefits to Former Personnel (13.0) | 0 | 0 | 0 |
| Subtotal Pay Costs | $81,610 | $82,673 | $1,063 |
| Travel & Transportation of Persons (21.0) | $3,653 | $3,712 | 58 |
| Transportation of Things (22.0) | 295 | 300 | 5 |
| Rental Payments to Others (23.2) | 62 | 62 | 1 |
| Communications, Utilities and Misc. Charges (23.3) | 609 | 619 | 10 |
| Printing and Reproduction (24.0) | 0 | 0 | 0 |
| Other Contractual Services: | |||
| Consultant Services (25.1) | 2,965 | 3,013 | 47 |
| Other Services (25.2) | 36,216 | 36,795 | 579 |
| Purchases from government accounts (25.3) | 140,903 | 142,956 | 2,053 |
| Operation and Maintenance of Facilities (25.4) | 1,840 | 1,840 | 0 |
| Operation and Maintenance of Equipment (25.7) | 3,346 | 3,400 | 54 |
| Subsistence and Support of Persons (25.8) | 0 | 0 | 0 |
| Subtotal Other Contractual Services | $185,271 | $188,004 | $2,733 |
| Supplies and Materials (26.0) | $9,390 | $9,528 | $138 |
| Subtotal Non-Pay Costs | $199,281 | $202,226 | $2,946 |
| Total Administrative Costs | $280,891 | $284,899 | $4,008 |
Details of Full-Time Equivalent Employment (FTE's)
| OFFICE/DIVISION | FY 2014 Actual |
FY 2015 Est. |
FY 2016 Est. |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Civilian | Military | Total | Civilian | Military | Total | Civilian | Military | Total | |
| Office of the Director | |||||||||
| Direct: | 55 | - | 55 | 56 | - | 56 | 56 | - | 56 |
| Reimbursable: | 1 | - | 1 | 1 | - | 1 | - | - | 1 |
| Total: | 56 | - | 56 | 57 | - | 57 | 57 | - | 57 |
| Division of Extramural Research | |||||||||
| Direct: | 105 | - | 105 | 106 | - | 106 | 106 | - | 106 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 105 | - | 105 | 106 | - | 106 | 106 | - | 106 |
| Division of Intramural Research | |||||||||
| Direct: | 322 | 7 | 329 | 324 | 7 | 331 | 324 | 7 | 331 |
| Reimbursable: | 6 | - | 6 | 6 | - | 6 | 6 | - | 6 |
| Total: | 328 | 7 | 335 | 330 | 7 | 337 | 330 | 5 | 337 |
| Office of Translational Research | |||||||||
| Direct: | 18 | - | 18 | 19 | - | 19 | 19 | - | 19 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 18 | - | 18 | 19 | - | 19 | 19 | - | 19 |
| Office of Clinical Research | |||||||||
| Direct: | 18 | - | 18 | 19 | - | 19 | 19 | - | 19 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 18 | - | 18 | 19 | - | 19 | 19 | - | 19 |
| Total | 525 | 7 | 532 | 528 | 7 | 535 | 528 | 7 | 535 |
Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
FTEs supported by funds from Cooperative Research and Development Agreements
| FISCAL YEAR | Average GS Grade |
|---|---|
| 2012 | 13.4 |
| 2013 | 12.0 |
| 2014 | 12.0 |
| 2015 | 12.0 |
| 2016 | 12.5 |
Detail of Positions
(Dollars in Thousands)
| GRADE | FY 2014 Actual |
FY 2015 Enacted |
FY 2016 President's Budget |
|---|---|---|---|
| Total, ES Positions | 1 | 1 | 1 |
| Total, ES Salary | 181,500 | 181,500 | 181,500 |
| GM/GS-15 | 34 | 34 | 34 |
| GM/GS-14 | 59 | 59 | 59 |
| GM/GS-13 | 85 | 86 | 86 |
| GS-12 | 55 | 56 | 56 |
| GS-11 | 31 | 31 | 31 |
| GS-10 | 4 | 4 | 4 |
| GS-9 | 26 | 26 | 26 |
| GS-8 | 17 | 17 | 17 |
| GS-7 | 13 | 13 | 13 |
| GS-6 | 2 | 2 | 2 |
| GS-5 | 0 | 0 | 2 |
| GS-4 | 1 | 1 | 1 |
| GS-3 | 2 | 2 | 2 |
| GS-2 | 0 | 0 | 0 |
| GS-1 | 0 | 0 | 0 |
| Subtotal | 329 | 331 | 331 |
| Grades established by Act of July 1, 1944 (42 U.S.C. 207): | 0 | 0 | 0 |
| Assistant Surgeon General | 0 | 0 | 0 |
| Director Grade | 3 | 3 | 3 |
| Senior Grade | 0 | 0 | 0 |
| Full Grade | 1 | 1 | 1 |
| Senior Assistant Grade | 1 | 1 | 1 |
| Assistant Grade | 0 | 0 | 0 |
| Subtotal | 5 | 5 | 5 |
| Ungraded | 0 | 0 | 0 |
| Total permanent positions | 334 | 336 | 336 |
| Total positions, end of year | 552 | 552 | 552 |
| Total full-time equivalent (F T E) employment, end of year | 532 | 535 | 535 |
| Average ES salary | 181,500 | 181,500 | 181,500 |
| Average GM/GS grade | 12.5 | 12.5 | 12.5 |
| Average GM/GS salary | 99,617 | 99,617 | 99,617 |
Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
